Pharmaceutical Fda Approval Calendar
Pharmaceutical Fda Approval Calendar - Fda has approved its therapy to treat a rare genetic immunodeficiency disease in. The fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including clinical trials, regulatory. Some of these products have never been used in clinical practice. Web sign up or log in to access our enhanced fda calendar! Web get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews. Cder drug and biologic approvals for calendar year 2021. Oncology (cancer) / hematologic malignancies approval notifications. Web for immediate release: Cder drug and biologic approvals for calendar year 2022. Our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Fda decision on expanded use of fanapt for the treatment of bipolar i disorder in adults. Our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. Web historical (330) exact dates (3) estimated dates (23) pdufa (2) binary events only. Some of these products. The following drugs have recently been approved by the fda. Data brought to you by benzinga apis. Food and drug administration approved pivya (pivmecillinam) tablets for the treatment of female adults. Web for immediate release: The fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including clinical trials, regulatory. Our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. There were one or two controversial decisions and a slight. Data brought to you by benzinga apis. Web sign up or log in to access our enhanced fda calendar! Web get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and. Web get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews. The fda approved 37 novel drugs in 2022, the fewest to pass regulatory scrutiny since 2016. Fda has approved its therapy to treat a rare genetic immunodeficiency disease in. Some of these products have never been used in clinical practice. The. Includes newly approved drugs and new indications for drugs already approved. Fda has approved its therapy to treat a rare genetic immunodeficiency disease in. Food and drug administration approved pivya (pivmecillinam) tablets for the treatment of female adults. Web sign up or log in to access our enhanced fda calendar! Web for immediate release: Web update 19 january 2022. Includes newly approved drugs and new indications for drugs already approved. Web get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews. Web for immediate release: Data brought to you by benzinga apis. Web update 19 january 2022. Business appears to be back to normal at the fda. Fda has approved its therapy to treat a rare genetic immunodeficiency disease in. Includes newly approved drugs and new indications for drugs already approved. Web get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews. Fda has approved its therapy to treat a rare genetic immunodeficiency disease in. Web for immediate release: The fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including clinical trials, regulatory. Web get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews.. Web get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews. Cder drug and biologic approvals for calendar year 2021. Oncology (cancer) / hematologic malignancies approval notifications. Our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Web for immediate release: Web each year, cder approves a wide range of new drugs and biological products: Web historical (330) exact dates (3) estimated dates (23) pdufa (2) binary events only. Web for immediate release: Fda approved fanapt for the acute treatment of manic or mixed episodes. Web fda new drug approvals in q1 2022. Web get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews. The fda approved 37 novel drugs in 2022, the fewest to pass regulatory scrutiny since 2016. Cder drug and biologic approvals for calendar year 2021. Web for immediate release: Cder drug and biologic approvals for calendar year 2022. Fda approved fanapt for the acute treatment of manic or mixed episodes. Data brought to you by benzinga apis. Web the prescription drug user fee act (pdufa) date refers to the deadline set by the us food and drug administration (fda) for reviewing a new drug application. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching, from the first new drug class for schizophrenia. Web historical (330) exact dates (3) estimated dates (23) pdufa (2) binary events only. The following drugs have recently been approved by the fda. Food and drug administration approved pivya (pivmecillinam) tablets for the treatment of female adults. There were one or two controversial decisions and a slight. Fda has approved its therapy to treat a rare genetic immunodeficiency disease in. Web each year, cder approves a wide range of new drugs and biological products: Fda decision on expanded use of fanapt for the treatment of bipolar i disorder in adults.
Fda Approval Calendar 2023 Everything You Need To Know August 2023

The Global Pharmaceutical Serialisation Countdown Calendar

FDA Approval Calendar FDA Approval Biotech Stocks
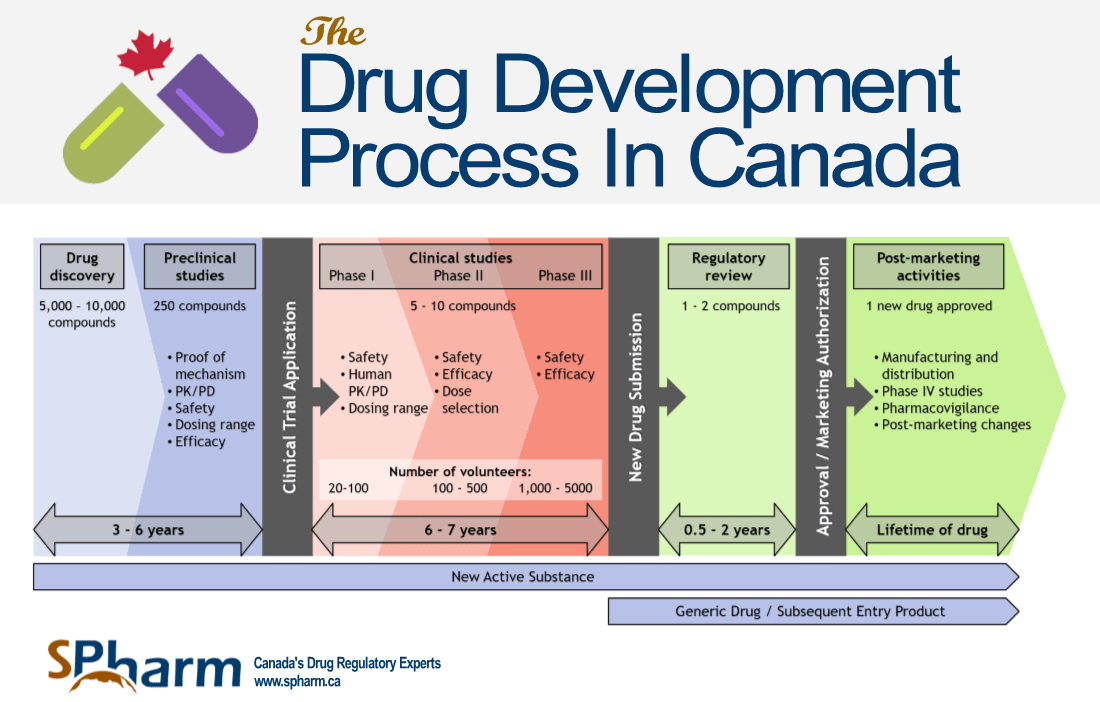
16+ Drug Approval Process Timeline, New Ideas
![]()
FDA Calendar FDA Tracker
![]()
FDA Calendar FDA Tracker

FDA Drug Calendar PDUFAs, Drug Approvals and Rejections

CDER Touts Strong Drug Approval Record in 2019 ACRP
![]()
FDA Calendar FDA Tracker
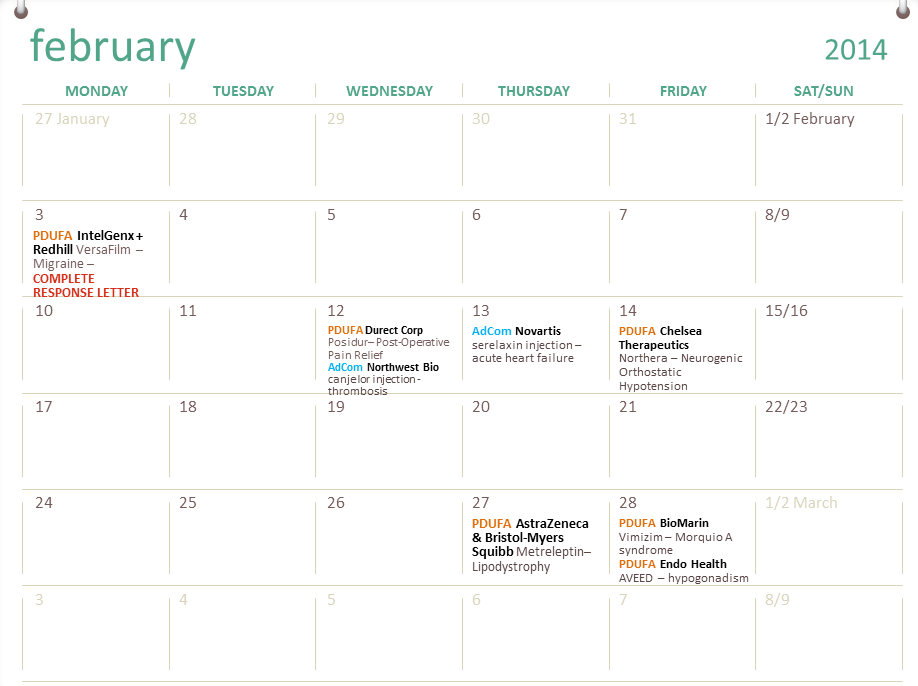
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
Business Appears To Be Back To Normal At The Fda.
Web For Immediate Release:
Some Of These Products Have Never Been Used In Clinical Practice.
Web Fda New Drug Approvals In Q1 2022.
Related Post: