How To Draw Orbital Diagram
How To Draw Orbital Diagram - To write the orbital diagram for the chlorine atom (cl) first we need to write the electron configuration for just cl. Web a p orbital which extends along the x axis is labeled a p x orbital. Web how to draw orbital diagrams. 16k views 2 years ago. Web by lee johnson. We build electron configurations by filling the lowest energy orbitals first then filling progressively higher energy orbitals. Examples of how to draw electron configuration. This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above. For example, the orbital diagram of li can be shown as: Orbital energy level diagram for the hydrogen atom with a single electron. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips. Web by lee johnson. An orbital is a space where a specific pair of electrons can be found. Web here are kepler’s three laws: These steps may then be. Typically, they only show the outermost electrons. Orbital diagrams must follow 3 rules: To write the orbital diagram for the chlorine atom (cl) first we need to write the electron configuration for just cl. For example, the orbital diagram of li can be shown as: Web this chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. Atoms & elements 4h 15m. This is known as the aufbau principal. Web drawing molecular orbital diagrams is one of the trickier concepts in chemistry. Web a p orbital which extends along the x axis is labeled a p x orbital. Web steps for drawing an orbital diagram. The aufbau principle, the pau. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips. So we fill subshells in the order 1s 2s 2p because 1s is the lowest energy, 2s is higher energy, and 2p is highest. For example, the orbital diagram of li can be shown as: To do that we need to find the number of. Web in orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down. Web a p orbital which extends along the x axis is labeled a p x orbital. So we. Web a p orbital which extends along the x axis is labeled a p x orbital. Web here are kepler’s three laws: You will also learn how to use hund'. The aufbau principle, the pau. This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above. The aufbau principle, the pau. We classified the different orbital into shells and sub shells to distinguish them more easily. We build electron configurations by filling the lowest energy orbitals first then filling progressively higher energy orbitals. Web steps for drawing an orbital diagram. A p orbital along the y axis is labeled p y and one along the z. To do that we need to find the number of. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips. This is known as the aufbau principal. Web how to draw orbital diagrams. We build electron configurations by filling. Atoms & elements 4h 15m. Orbital diagrams must follow 3 rules: The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips. The sun's center is always located at one focus of the ellipse. Note that the difference in energy. This is known as the aufbau principal. To do that we need to find the number of. Web this video goes over how to properly draw orbital diagrams for an element, after determining the electron configuration. Each planet's orbit about the sun is an ellipse. The arrow shows a qualitative representation of increasing orbital energy. A p orbital along the y axis is labeled p y and one along the z axis is a p z orbital. To write the orbital diagram for the chlorine atom (cl) first we need to write the electron configuration for just cl. Orbital energy level diagram for the hydrogen atom with a single electron. From this diagram, calculate the bond order for o 2. Web here are kepler’s three laws: This is a way of showing the electron configuration of the atom. Remember, we can use the periodic table to help us. Web the objective of this wiki is to provide readers with the fundamental steps in constructing simple homonuclear and heteronuclear diatomic molecular orbital diagrams. These steps may then be extrapolated to construct more difficult polyatomic diagrams. Atoms & elements 4h 15m. Each box corresponds to one orbital. The sun's center is always located at one focus of the ellipse. Write out the electron configuration to determine which orbitals are filled. Examples of how to draw electron configuration. Web this video goes over how to properly draw orbital diagrams for an element, after determining the electron configuration. It explains how to write the orbital diagram n.
Shapes of Atomic Orbitals — Overview & Examples Expii

Molecular Orbital Diagrams simplified by Megan Lim Medium
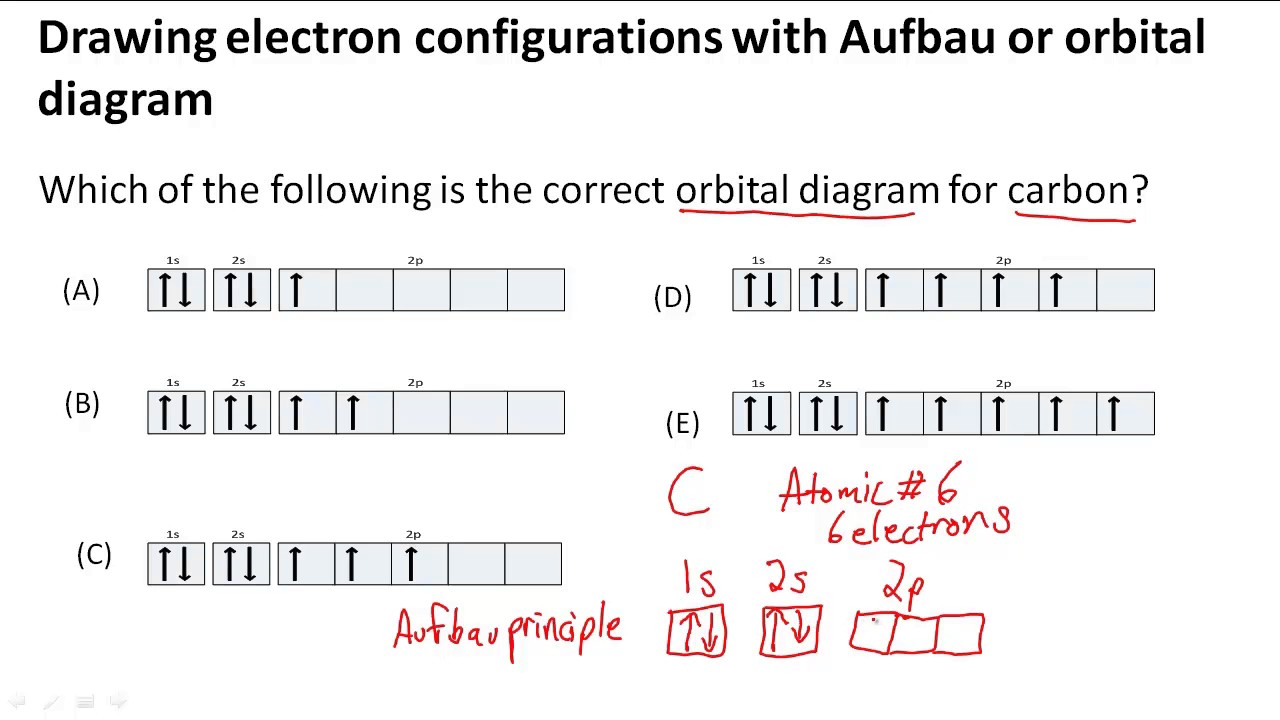
Drawing electron configurations with Aufbau/orbital diagram YouTube

P Orbital diagram How to draw orbital diagram of Phosphorus YouTube
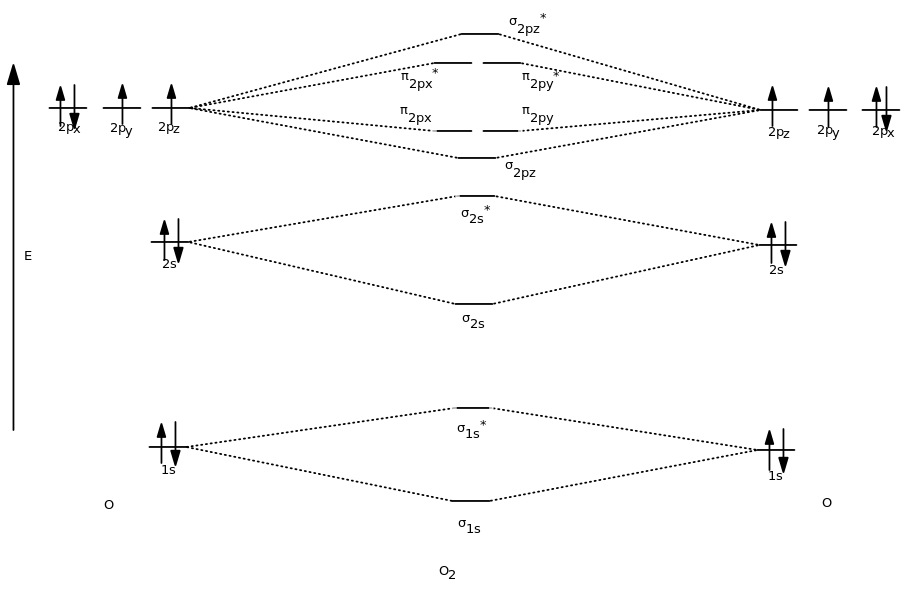
Drawing Atomic and Molecular Orbitals Diagrams for Molecules Organic

Radial and Angular Parts of Atomic Orbitals Chemistry LibreTexts

Shapes of Orbitals and their Types Chemistry Skills

How to Draw Shapes of Orbitals

Atomic orbitals explained polizhuge

How To Draw A Molecular Orbital Diagram Elevatorunion6
Each Planet's Orbit About The Sun Is An Ellipse.
The Aufbau Principle, The Pau.
To Do That We Need To Find The Number Of.
This Is Done By First Determining The Subshell (S,P,D, Or F) Then Drawing In Each Electron According To The Stated Rules Above.
Related Post: