How To Draw An Ionic Bond
How To Draw An Ionic Bond - Web ionic bonds are formed when positively and negatively charged ions are held together by electrostatic forces. 6.7k views 7 years ago edexcel. This chemistry video explains how to draw the lewis structures of ionic compounds. A dot and cross diagram is one way to model the transfer of electrons that occurs during this process. Web shows how to draw lewis dot structures for ionic compounds. Magnesium has two electrons in its outer shell, oxygen has six. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. The octet rule refers to the tendency of atoms to. Web there are many types of chemical bonds and forces that bind molecules together. Swap the crosses for dots in one of your diagrams. These grades are the stepping stone to your future. The octet rule refers to the tendency of atoms to. Show how electrons are transferred in ionic bonding. Draw the outer shell of each atom. (note that we denote ions with brackets around the structure, indicating the. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Show how electrons are transferred in ionic bonding. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web draw a diagram, with outer electrons only, to show how the electrons are. Swap the crosses for dots in one of your diagrams. Want to join the conversation? (note that we denote ions with brackets around the structure, indicating the. Draw the electron configuration diagram for each atom. Draw the outer shell of each atom. Ionic bonds require at least one electron donor and one electron acceptor. Draw lewis structures for ionic compounds. Draw dot and cross diagrams for ionic compounds. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Web there are many types of chemical bonds and forces that bind molecules together. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. The octet rule refers to the tendency of atoms to. Draw the electron configuration diagram for each atom. 6.7k views 7 years ago edexcel. Web shows how to draw lewis. 292k views 3 years ago new ap & general chemistry video playlist. Draw the electron configuration diagram for each atom. Swap the crosses for dots in one of your diagrams. Draw dot and cross diagrams for ionic compounds. Electrostatics explains why this happens: Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Web ionic bonds are formed when positively and negatively charged ions are held together by electrostatic forces. Magnesium has two electrons in its outer shell, oxygen has six.. The octet rule refers to the tendency of atoms to. During ionic bonding the atoms form ions by magnesium has two electrons in its outer gaining or losing electrons to obtain a full outer shell, oxygen has six. Ionic bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom following the octet rule. Want. 6.7k views 7 years ago edexcel. Web shows how to draw lewis dot structures for ionic compounds. Draw lewis structures for ionic compounds. A dot and cross diagram is one way to model the transfer of electrons that occurs during this process. Draw the outer shell of each atom. This chemistry video explains how to draw the lewis structures of ionic compounds. A dot and cross diagram is one way to model the transfer of electrons that occurs during this process. Web shows how to draw lewis dot structures for ionic compounds. Activities 1 and 2 introduce ionic bonding dot and cross diagrams in a format that allows learners. Web 224k views 5 years ago. Web i want to help you achieve the grades you (and i) know you are capable of; Electrostatics explains why this happens: A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Want to join the conversation? Web drawing dot and cross diagrams. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Draw dot and cross diagrams for ionic compounds. A dot and cross diagram is one way to model the transfer of electrons that occurs during this process. Draw the outer shell of each atom. The two most basic types of bonds are characterized as either ionic or covalent. Draw lewis structures for ionic compounds. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. During ionic bonding the atoms form ions by magnesium has two electrons in its outer gaining or losing electrons to obtain a full outer shell, oxygen has six. Web shows how to draw lewis dot structures for ionic compounds. Web there are many types of chemical bonds and forces that bind molecules together.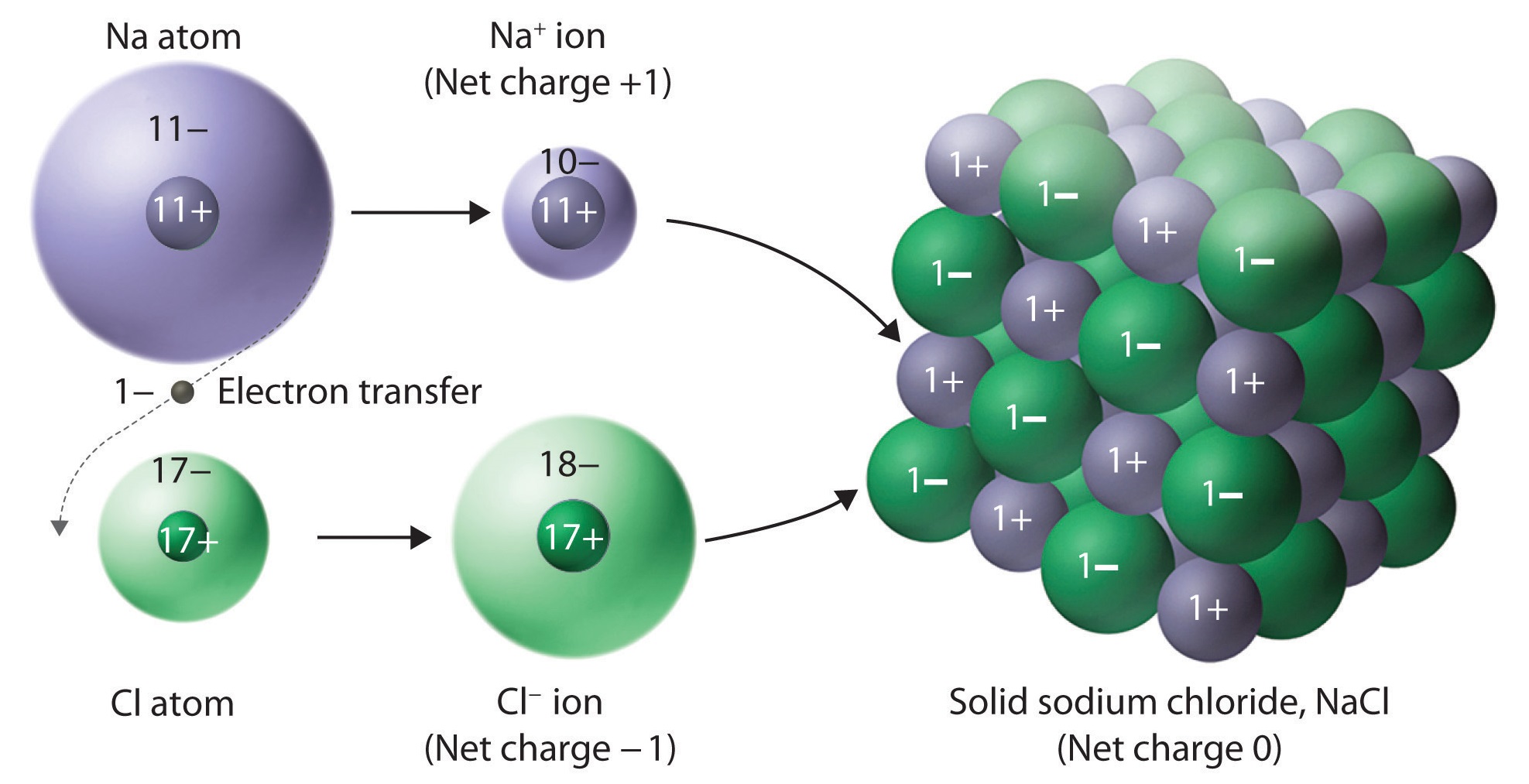
Ionic Solids Chemistry LibreTexts
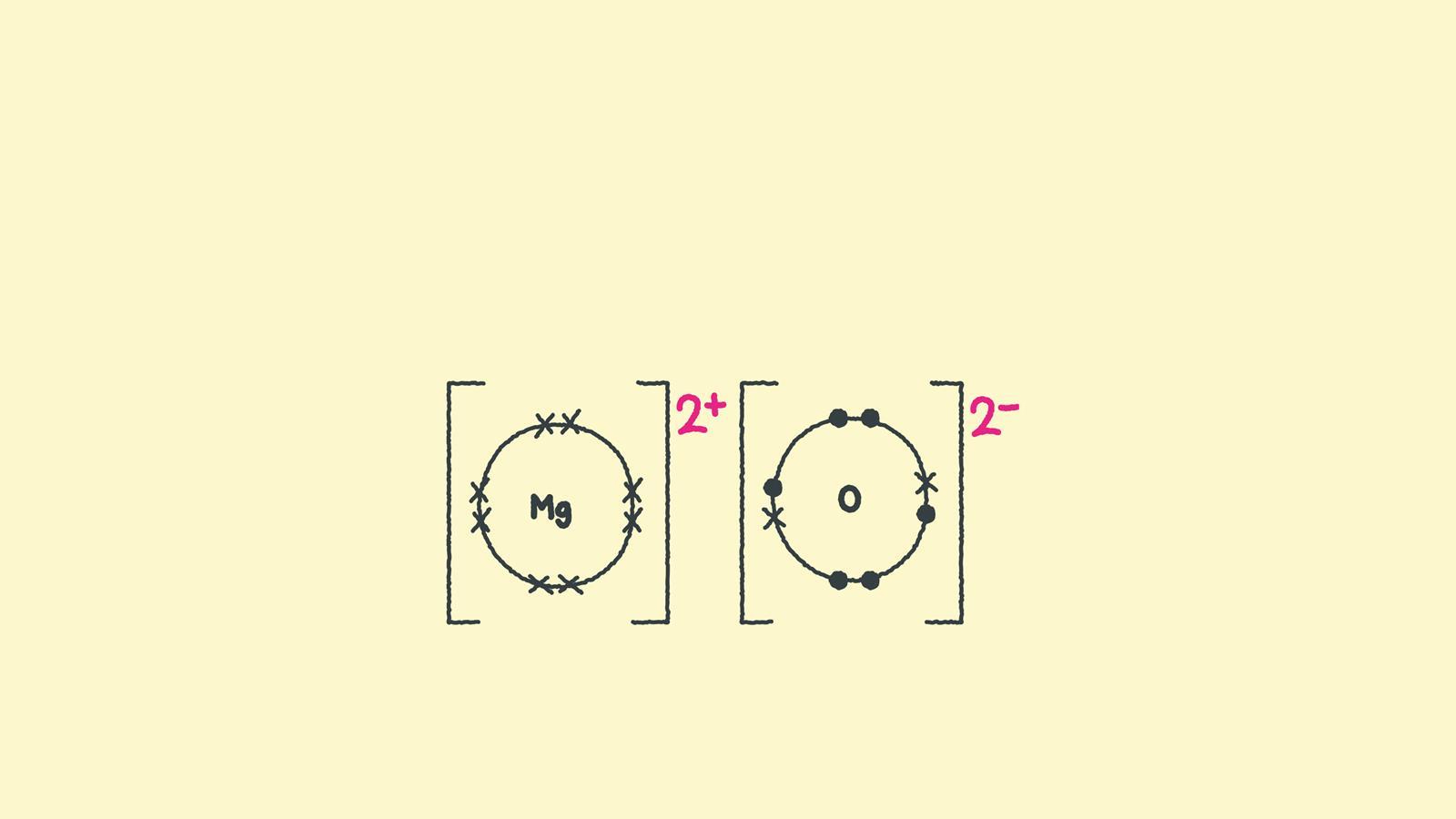
How to draw ionic bonding dot and cross diagrams Feature RSC Education
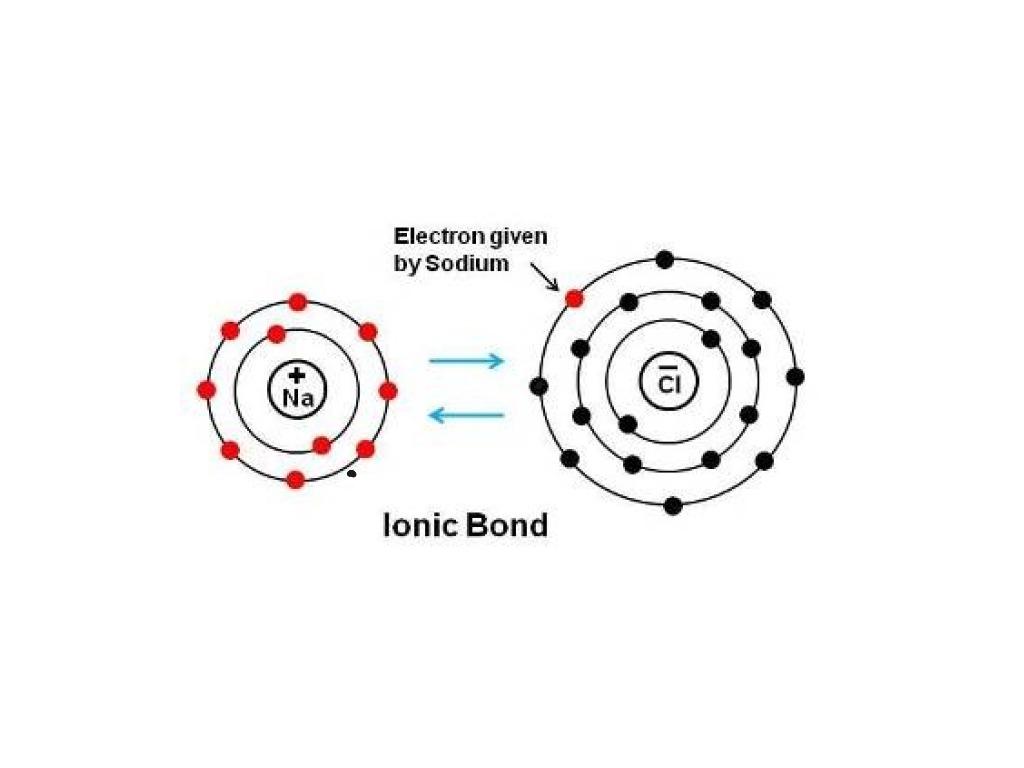
Ionic bond Science, Chemistry, Chemical Bonds ShowMe
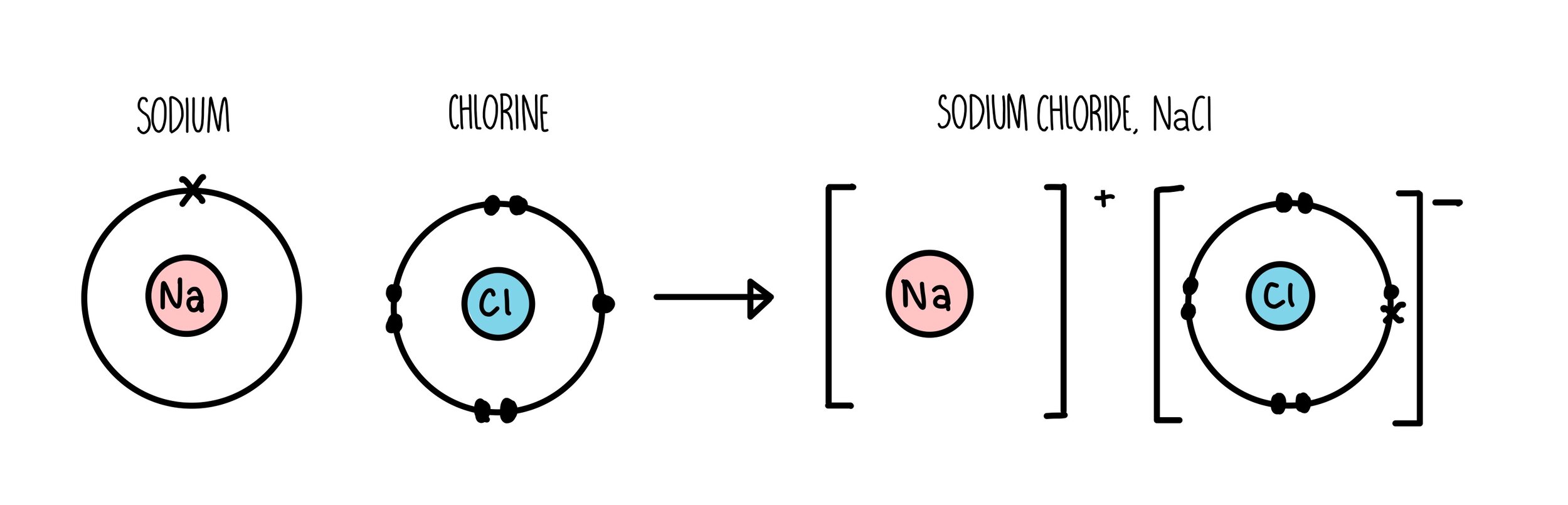
Ionic Bonding — the science hive

ionic bond Definition, Properties, Examples, & Facts Britannica
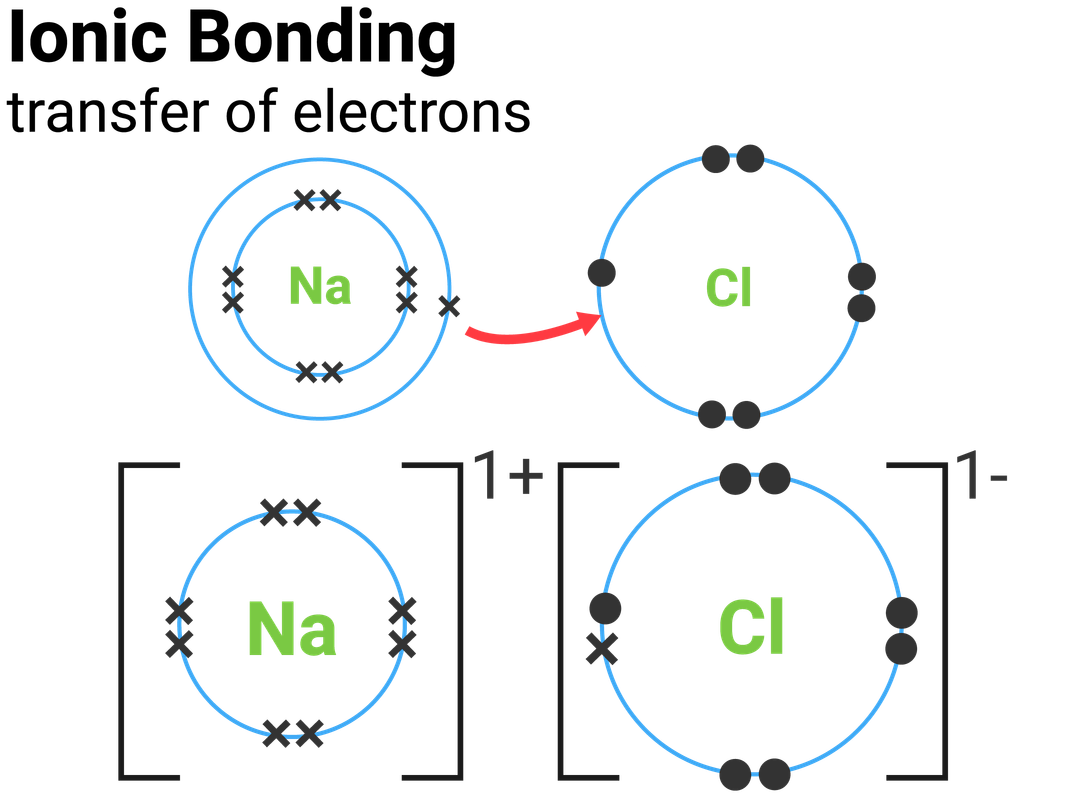
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk

Ionic Bonding GCSE Science Chemistry Get To Know Science YouTube
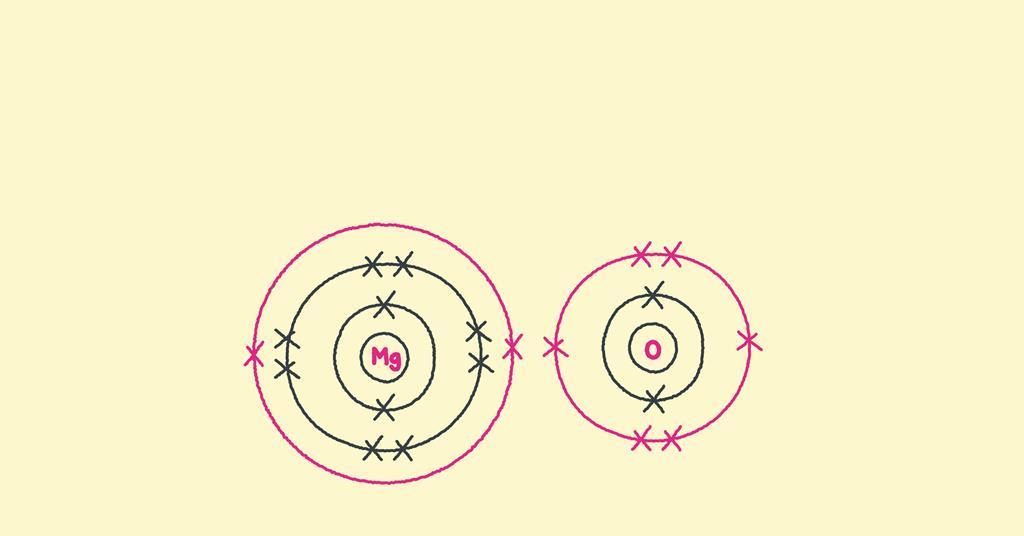
How to draw ionic bonding dot and cross diagrams Feature RSC Education
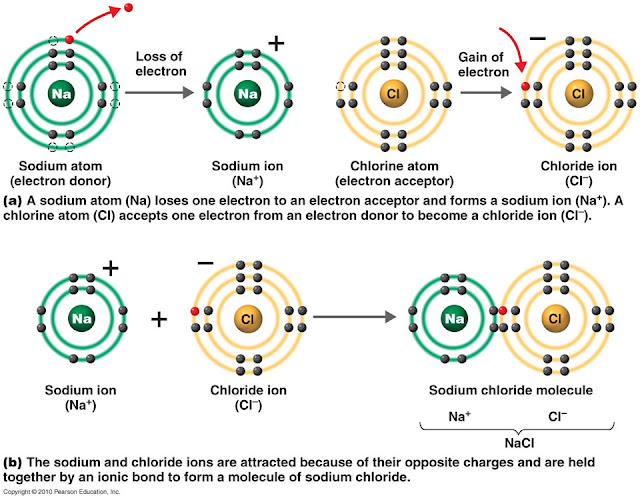
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
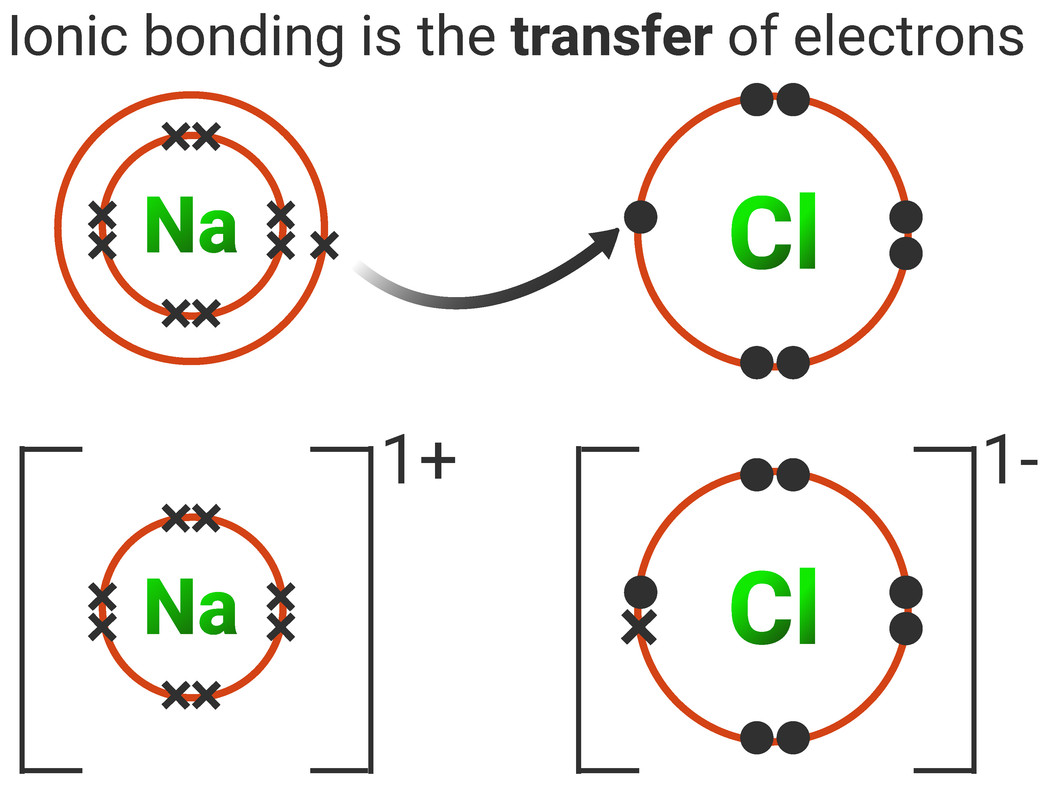
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk
Once They Have Mastered Electron Configuration Diagrams, Show Your Learners How They Can Adapt Them To Show Structure And Bonding In Covalent.
Ionic Bonds Require At Least One Electron Donor And One Electron Acceptor.
These Grades Are The Stepping Stone To Your Future.
Swap The Crosses For Shell.
Related Post: