How To Draw A Covalent Bond
How To Draw A Covalent Bond - You can tell from its formula that it is not an ionic compound; Arrange the atoms to show specific bonds. Web representing a covalent bond using lewis structures. Draw lewis structures depicting the bonding in simple molecules. The video covers the basic lewis structures. Let us illustrate a covalent bond by using h atoms, with the understanding that h atoms need only two electrons to fill the first shell. Web a covalent bond is a type of chemical bond characterized by two atoms sharing valence electrons. It is not composed of a. Memorize numerical prefixes used in covalent nomenclature. What is the organic periodic table? One substance mentioned previously was water ( h2o h 2 o ). Web covalent bonds involve shared electron pairs between atoms. In this video you’ll learn how to draw lewis dot structures for covalent compounds. To describe how a covalent bond forms. To apply the octet rule to covalent compounds. Web one line is a single bond with 2 bonding electrons, two lines is a double bond with 4 bonding electrons, and three lines is a triple bond with 6 bonding electrons. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Determine the total number of valence electrons in the. By the end of this section, you will be able to: Atoms share the same number of pairs needed to. Web a covalent bond is a type of chemical bond characterized by two atoms sharing valence electrons. To know what types of elements bond to form covalent compounds. Memorize numerical prefixes used in covalent nomenclature. Electronegativity is a measure of how strongly an atom attracts electrons from another atom in a chemical bond and this value is governed by where the particular atom is located in the periodic table (francium is the least electronegative element while fluorine is the most electronegative). It is not composed of a. Atoms share the same number of pairs needed. By the end of this section, you will be able to: A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Each atom contributes one electron to the bond. Memorize numerical prefixes used in covalent nomenclature. (recall that the number of valence electrons is indicated by the position of the element. Each atom contributes one electron to the bond. Web how to draw covalent bonding molecules. Web this is called a covalent bond. What is the organic periodic table? Arrange the atoms to show specific bonds. In this video you’ll learn how to draw lewis dot structures for covalent compounds. Each atom contributes one electron to the bond. Web one line is a single bond with 2 bonding electrons, two lines is a double bond with 4 bonding electrons, and three lines is a triple bond with 6 bonding electrons. Covalent bonds form when two atoms. To know what types of elements bond to form covalent compounds. In this video you’ll learn how to draw lewis dot structures for covalent compounds. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Add together the valence electrons from each atom. Illustrate covalent bond formation with lewis electron dot. By the end of this section, you will be able to: Usually, sharing electrons gives each atom a full valence shell and makes the resulting compound more stable than its constituent atoms. Web and introductory video for my general chemistry class (goes along with notes packet #9) showing lewis dot structures for simple molecules involving single, d. Web covalent bonds. Web draw lewis structures for covalent compounds. Only when two atoms of the same element form a covalent bond are the shared electrons actually shared equally between the atoms. It is not composed of a. Determine the total number of valence electrons in the molecule or ion. 1.31 explain the formation of simple molecular, covalent substances, using dot and cross. Atoms share the same number of pairs needed to. To know what types of elements bond to form covalent compounds. The following procedure can be used to construct lewis electron structures for more complex molecules and ions. Determine the total number of valence electrons in the molecule or ion. Add together the valence electrons from each atom. One substance mentioned previously was water ( h2o h 2 o ). To describe how a covalent bond forms. 1.31 explain the formation of simple molecular, covalent substances, using dot and cross diagrams,. In this video you’ll learn how to draw lewis dot structures for covalent compounds. Each atom contributes one electron to the bond. (recall that the number of valence electrons is indicated by the position of the element in the periodic table.) 2. Web one line is a single bond with 2 bonding electrons, two lines is a double bond with 4 bonding electrons, and three lines is a triple bond with 6 bonding electrons. Web covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or fairly similar ionization energies and electron affinities). To apply the octet rule to covalent compounds. Web draw lewis structures for covalent compounds. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons.
Covalent Bonding The Science and Maths Zone

Covalent Bonding The Science and Maths Zone
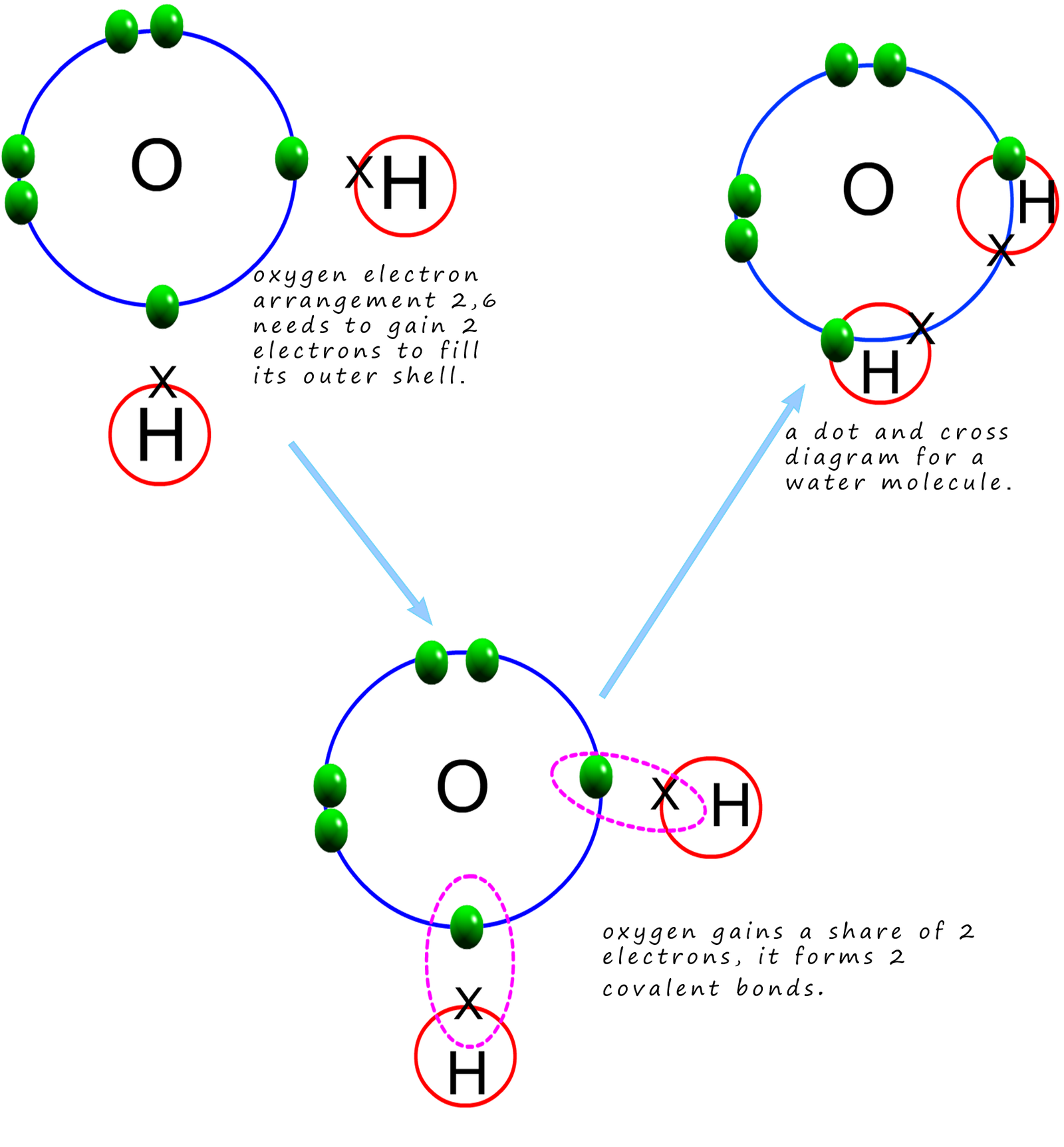
Covalent bonding
Covalent bond (covalency) and its type Overall Science
The Covalent Bond CK12 Foundation

How is a covalent bond formed

Covalent Bond Definition, Types, and Examples
The Covalent Bond CK12 Foundation

Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk

Covalent Bond Biology Dictionary
4.7K Views 7 Years Ago 1D:
Web This Is Called A Covalent Bond.
Any Reference To H2O, Nh3, Ch4, O2, N2 Or Co2 Bonding Is Higher Tier.** We Can Use Dot And Cross Diagrams To Show How A Pair Of.
0:08 Introduction 0:39 H2 1:25 Hcl.
Related Post:

