Drawing Orbitals
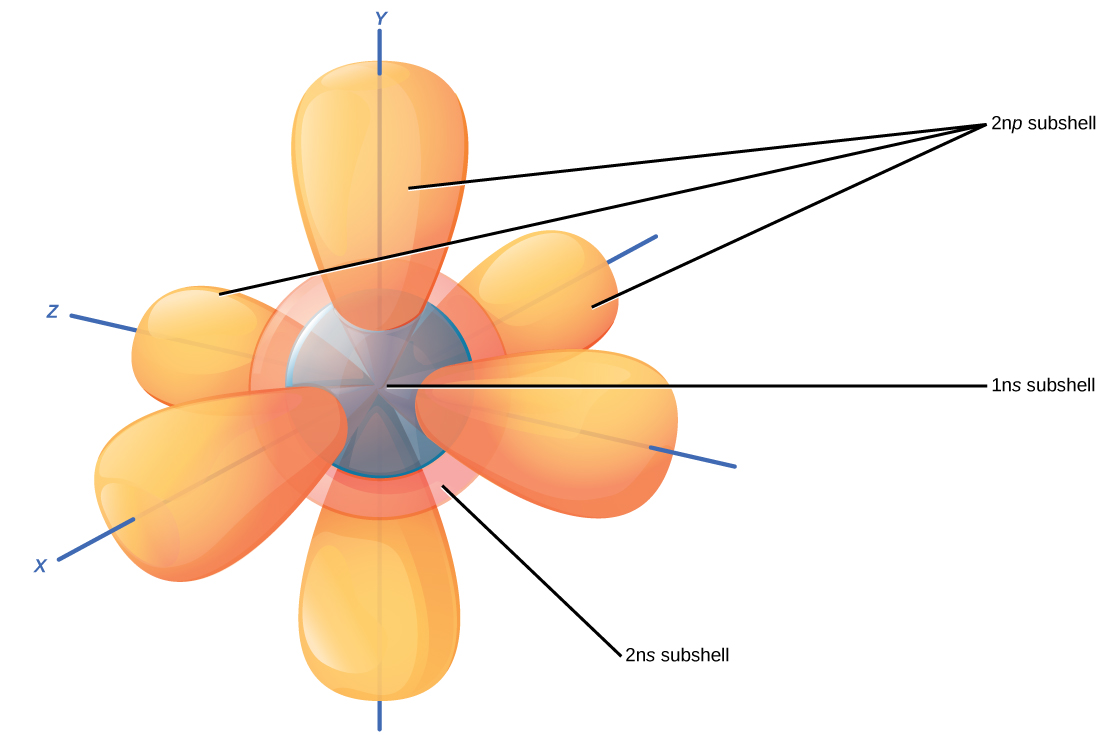
Drawing Orbitals - Web an orbital is the quantum mechanical refinement of bohr’s orbit. Web get out your pencil (and eraser) because we are about to learn how to draw atomic orbitals. This article will explore the basics of how to draw each type of diagram, and important rules to follow in their construction. Of the four, we’ll be concerned primarily with s and p orbitals because these are the most common in organic and biological chemistry. The lowest energy orbitals are filled before the higher energy orbitals ( aufbau principle) each orbital gets one electron first, before adding the second electron to the orbital ( hund’s rule) only 2 electrons, of opposite spins, can occupy each orbital ( pauli exclusion principle) This includes all hybrid orbitals. Web an orbital is a space where a specific pair of electrons can be found. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Web draw any atom: Any molecule can be formed using linear combination of atomic orbitals (lcao). Web an orbital is the quantum mechanical refinement of bohr’s orbit. There are four different kinds of orbitals, denoted s, p, d, and f, each with a different shape. Do you want more practice? Web what do orbitals look like? Any atom with the principal orbital quantum number, n=30. Boundary surface diagrams of the constant probability density for different orbitals help us understand the shape of orbitals. How might one draw atomic and molecular orbital diagrams? A p orbital along the y axis is labeled p y and one along the z axis is a p z orbital. Web atomic orbitals are the wavefunctions which are solutions of the. With oxygen, you know that the atomic orbital potential energies go in the following order: So the atomic orbital diagram is simply those orbitals in that order of energy. · 4 · nov 17 2015. Do you want more practice? Web the four chemically important types of atomic orbital correspond to values of \(\ell = 0\), \(1\), \(2\), and \(3\). Web the three rules for creating orbital diagrams. Orbitals with \(\ell = 0\) are s orbitals and are spherically symmetrical, with the greatest probability of finding the electron occurring at the nucleus. Orbital diagrams are a visual way to show where the electrons are located within an atom. Web this video goes over how to properly draw orbital diagrams for. Boundary surface diagrams of the constant probability density for different orbitals help us understand the shape of orbitals. This is also due to the history when they were discovered. Any atom with the principal orbital quantum number, n=30. So the atomic orbital diagram is simply those orbitals in that order of energy. The bohr model shows the atom as a. Web this chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. We classified the different orbital into shells and sub shells to distinguish them more easily. Web an orbital is a space where a specific pair of electrons can be found. Orbital diagrams must follow 3 rules: Below are dot density diagrams, boundary surface diagrams, and. I will use oxygen ( o2(g)) as an example. In the previous section we learned that electrons have wave/particle duality, exist in orbitals that are defined by the schrödinger wave equation that involves the complex coordinate system and imaginary numbers, and that they can be defined by quantum numbers n, l and m l. Web © 2024 google llc. Ensure. A p orbital along the y axis is labeled p y and one along the z axis is a p z orbital. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Web © 2024 google llc. It explains how to write the orbital diagram. Orbital diagrams must follow 3 rules: Orbital diagrams are a visual way to show where the electrons are located within an atom. Web this chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. Web what do orbitals look like? Web the three rules for creating orbital diagrams. Web atomic orbitals are the wavefunctions which are solutions of the schroumldinger equation for the hydrogen atom the subset of atomic orbitals and are plotted in three dimensions to exhibit their characteristic shapes the orbitals are drawn by showing their boundary surfaces in the second view and signs are attached to the. This is also due to the history when. So the atomic orbital diagram is simply those orbitals in that order of energy. In the previous section we learned that electrons have wave/particle duality, exist in orbitals that are defined by the schrödinger wave equation that involves the complex coordinate system and imaginary numbers, and that they can be defined by quantum numbers n, l and m l. Although such drawings show the relative sizes of the orbitals, they do not normally show the spherical nodes in the 2 s and 3 s orbitals. Web this wave function also helps us in drawing boundary surface diagrams. Let's learn about the shapes of atomic orbitals in. I will use oxygen ( o2(g)) as an example. You will also learn how to use hund'. Web get out your pencil (and eraser) because we are about to learn how to draw atomic orbitals. The bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun. Orbitals can be represented as boxes with the electrons depicted with arrows. Web atomic orbitals are the wavefunctions which are solutions of the schroumldinger equation for the hydrogen atom the subset of atomic orbitals and are plotted in three dimensions to exhibit their characteristic shapes the orbitals are drawn by showing their boundary surfaces in the second view and signs are attached to the. Boundary surface diagrams of the constant probability density for different orbitals help us understand the shape of orbitals. Any atom with the principal orbital quantum number, n=30. A p orbital along the y axis is labeled p y and one along the z axis is a p z orbital. Orbital diagrams must follow 3 rules: 2.8k views 8 months ago physical chemistry.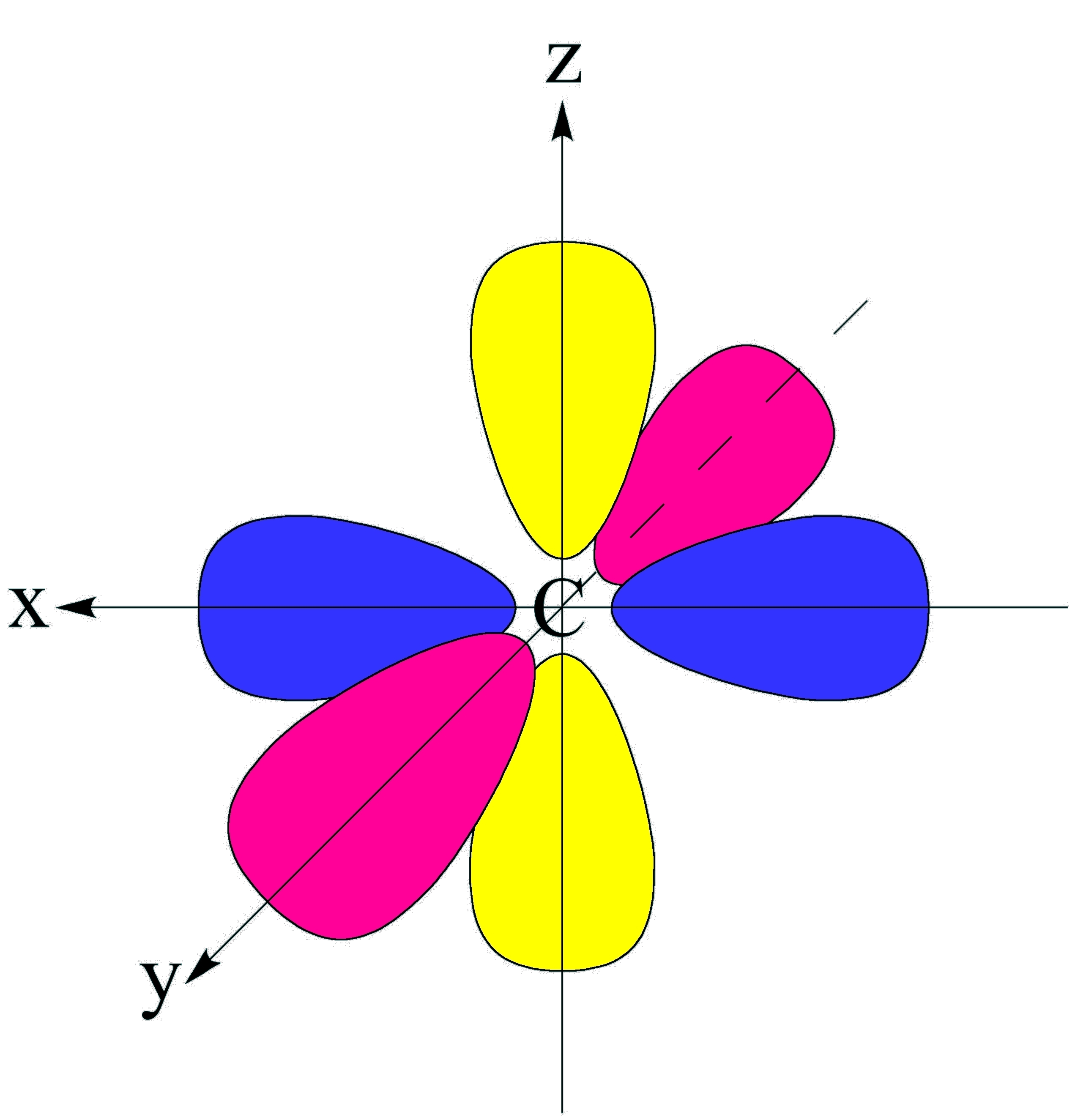
Illustrated Glossary of Organic Chemistry Orbital
Shapes of Orbitals and their Types Chemistry Skills
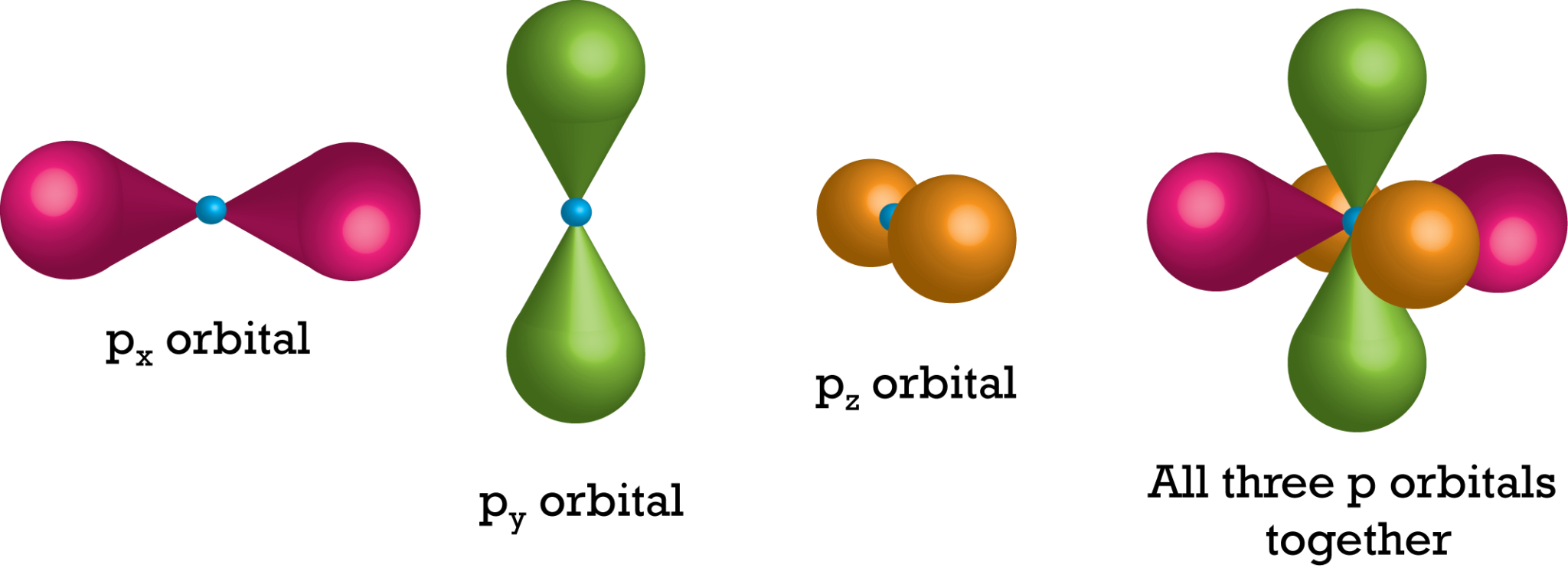
Electrons Biology for Majors I

Shapes of Atomic Orbitals — Overview & Examples Expii

Shapes of Atomic Orbitals — Overview & Examples Expii

How to Draw Shapes of Orbitals

3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts

Orbitals 2. Shape Biochemistry Flashcards ditki medical and

Radial and Angular Parts of Atomic Orbitals Chemistry LibreTexts

Atom Orbitals Chart
Below Are Dot Density Diagrams, Boundary Surface Diagrams, And A Rotating Image.
Orbital Diagrams Are A Visual Way To Show Where The Electrons Are Located Within An Atom.
· 4 · Nov 17 2015.
Web The Three Rules For Creating Orbital Diagrams.
Related Post: