Drawing Covalent Compounds Worksheet
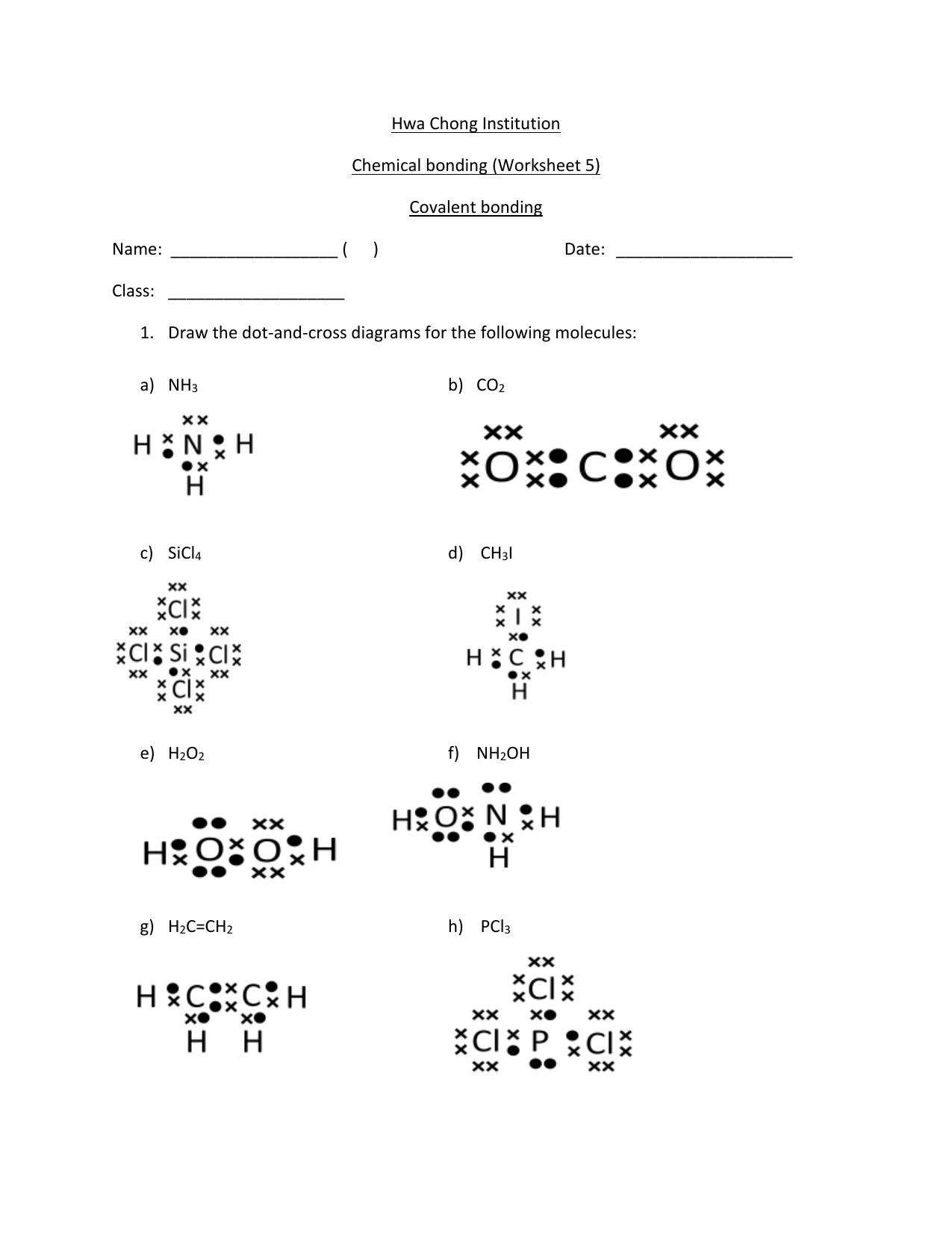
Drawing Covalent Compounds Worksheet - 7) ammonia (nitrogen trihydride) nh3. For the following molecules or ions (where the central atom is underlined): Web unit 4 (covalent compounds) 1. 0132525887_chem_wkbk_ch 08.indd 97 3/24/10 7:10:56 pm. The following procedure can be used to draw lewis structure for simple molecules. The compound is often added to otherwise odorless fuels such as natural gas to help warn of gas leaks. The following procedure can be used to construct lewis electron structures for more complex molecules and ions. Draw the lewis structures for each of the following molecules. The following procedure can be used to construct lewis electron structures for more complex molecules and ions. Web covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their outer shell for at least part of the time. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Which of the following correctly completes the lewis. The following procedure can be used to construct lewis electron structures for more complex molecules and ions. The skeletal structure of ethanethiol is shown below. Write the electron. The skeletal structure of ethanethiol is shown below. Web draw lewis structures for covalent compounds. Web draw lewis structures for covalent compounds. You should consult the lewis structure rules and a. 21) so 3 sulfur trioxide 22) n 2 s dinitrogen sulfide 23) ph 3 phosphorus trihydride 24) bf 3 boron trifluoride 25) p 2 br 4 diphosphorus tetrabromide 26). Web 30 7.3 lewis structures and covalent compounds. Web covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their outer shell for at least part of the time. Web draw lewis structures for covalent compounds. A molecule is a neutral group of atoms joined together by covalent bonds. Determine the. Draw the lewis dot structures for each of the following molecules: Draw lewis structures for the following covalent compounds: 21) so 3 sulfur trioxide 22) n 2 s dinitrogen sulfide 23) ph 3 phosphorus trihydride 24) bf 3 boron trifluoride 25) p 2 br 4 diphosphorus tetrabromide 26) co carbon monoxide 27) sio 2 silicon dioxide 28) sf 6 sulfur. Draw lewis structures for the following covalent compounds: Web covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their outer shell for at least part of the time. Write the electron dot structure (lewis dot structure) for covalent compounds or ions. Ch 2 br 2 d. The following procedure can. This worksheet clearly explains how to draw dot and cross diagrams for covalent compounds, using cl2 as an example. Web 30 7.3 lewis structures and covalent compounds. Web draw lewis structures for covalent compounds. Given the name of a covalent compound, write its formula. A) bef 2 b) bcl 3 c) ccl 4 d) pbr 5 e) si 6. Use electronegativity to determine the polarity of a bond or molecule. Write the electron dot structure (lewis dot structure) for covalent compounds or ions. You should consult the lewis structure rules and a. The following procedure can be used to construct lewis electron structures for more complex molecules and ions. Illustrate covalent bond formation with lewis electron dot diagrams. Draw lewis structures for the following covalent compounds: Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. A molecule is a neutral group of atoms joined together by covalent bonds. The following procedure can be used to construct lewis electron structures for more complex molecules. How is ionic bonding different from covalent bonding? Draw the electron dot structure. Draw lewis structures for the following covalent compounds: Given the formula of a covalent compound, write its correct name; Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Give the name of the electronic arrangement and the name for the molecular geometry for each of the species in question #1. Circle the unpaired electrons that will be shared between the elements. What is the difference between a molecule and a formula unit? Molecular compounds a molecular formula shows how many atoms of each element a molecule contains. Draw. Web write the formulas for the following covalent compounds: 7) ammonia (nitrogen trihydride) nh3. Illustrate covalent bond formation with lewis electron dot diagrams. How is ionic bonding different from covalent bonding? 2 sample questions showing how to properly draw covalent bond diagrams. Draw the best lewis dot structures for each of the following species. Ch 2 br 2 d. Determine the total number of valence electrons in the molecule or ion. Circle the unpaired electrons that will be shared between the elements. 21) so 3 sulfur trioxide 22) n 2 s dinitrogen sulfide 23) ph 3 phosphorus trihydride 24) bf 3 boron trifluoride 25) p 2 br 4 diphosphorus tetrabromide 26) co carbon monoxide 27) sio 2 silicon dioxide 28) sf 6 sulfur hexafluoride 29) nh 3 ammonia 30) no 2 nitrogen dioxide The skeletal structure of ethanethiol is shown below. The following procedure can be used to construct lewis electron structures for more complex molecules and ions. Web draw lewis structures for covalent compounds. A) bef 2 b) bcl 3 c) ccl 4 d) pbr 5 e) si 6. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Why do hydrogen atoms not follow the octet rule when they form covalent bonds?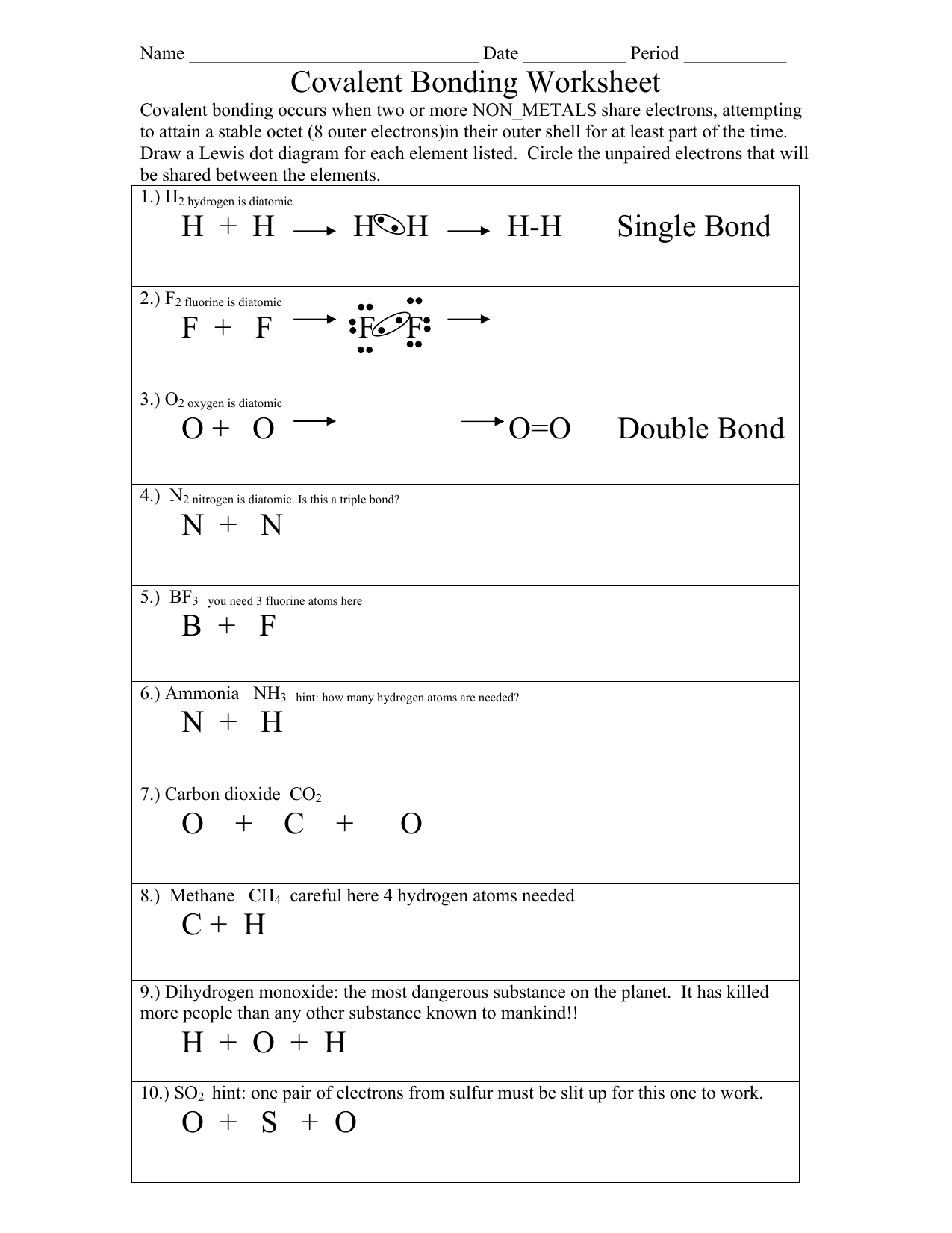
Covalent Bonding Worksheet

Naming Covalent Compounds Worksheet
Molecular Models of Covalent Compounds Worksheet

Naming Covalent Compounds Made Easy With This Worksheet Style Worksheets
Covalent Bonding Worksheet —

Naming Covalent Compounds Worksheet Answers —
Covalent Bonding Practice Worksheet Answers Worksheet Now

Naming Covalent Compounds Type Iii Worksheet 5 1 Free Worksheets Samples

Drawing Covalent Compounds Worksheet Answers Free Worksheets Samples

Covalent compound worksheet
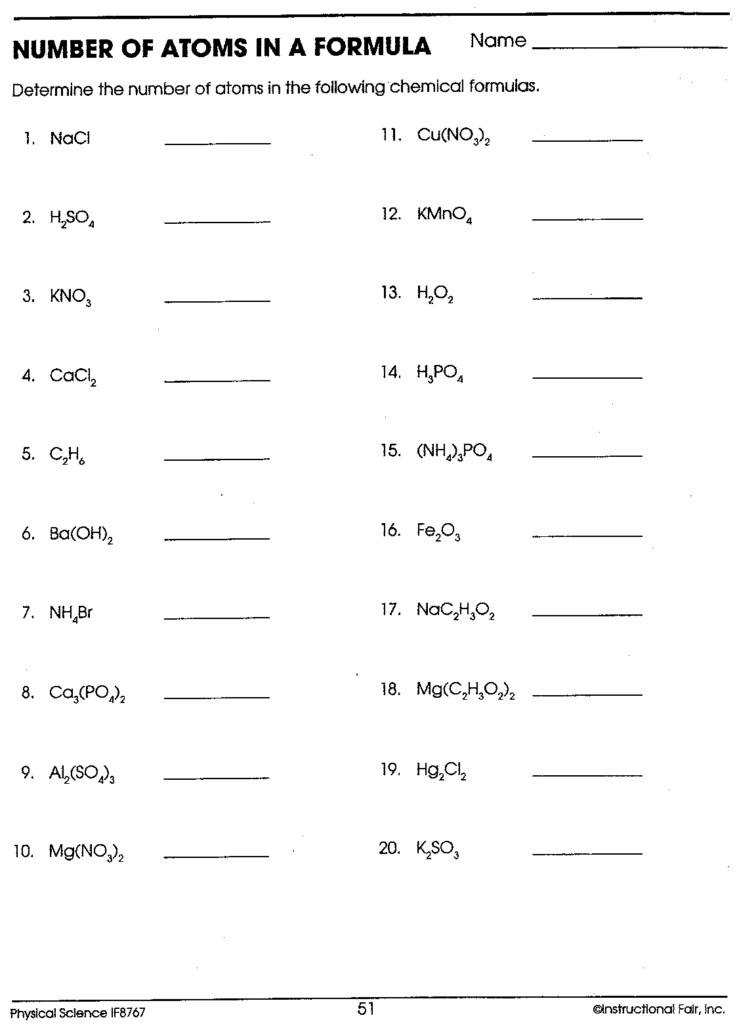
Complete In The Following Table:
Web Draw The Best Lewis Dot Structure For Each Of The Following Species.
For The Following Molecules Or Ions (Where The Central Atom Is Underlined):
Web Draw Lewis Structures For Covalent Compounds.
Related Post:
