Draw The Electron Configuration For A Neutral Atom Of Iron
Draw The Electron Configuration For A Neutral Atom Of Iron - Thus, it is simple to determine the charge on such a negative ion: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web electron configurations describe where electrons are located around the nucleus of an atom. Energy this problem has been solved! Note that the total number of electrons in the neutral atom adds up to the atomic number, so 2+2+6+2+6 = 18, which is the atomic number of ar. Web therefore the iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. 1 s 2 2 s 2 2 p 3. Web iron ion (fe 2+, fe 3+) electron configuration. Web for example, [ne] represents the 1s 2 2s 2 2p 6 electron configuration of neon (z = 10), so the electron configuration of sodium, with z = 11, which is 1s 2 2s 2 2p 6 3s 1, is written as [ne]3s 1: Web so, [ar] can be written instead of 1s^2 2s^2 2p^6 3s^2 3p^6. Thus, it is simple to determine the charge on such a negative ion: The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. Web therefore the iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Energy this problem. Note that when writing the electron configuration for an atom like fe, the 3d is usually written before the 4s. Otherwise, write the order of the energy levels with electron configuration chart: Web so, [ar] can be written instead of 1s^2 2s^2 2p^6 3s^2 3p^6. The charge is equal to the number of electrons that must be gained to fill. Web for example, [ne] represents the 1s 2 2s 2 2p 6 electron configuration of neon (z = 10), so the electron configuration of sodium, with z = 11, which is 1s 2 2s 2 2p 6 3s 1, is written as [ne]3s 1: Web iron ion (fe 2+, fe 3+) electron configuration. Web draw the electron configuration for a. Web most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill its outer s and p orbitals, thereby reaching the electron configuration of the next noble gas. Energy this problem has been solved! Web what is the electron configuration of: Note that the total number of electrons in the neutral atom adds up to the. Note that when writing the electron configuration for an atom like fe, the 3d is usually written before the 4s. Web draw the electron configuration for a neutral atom of iron. Web therefore the iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Web iron ion (fe 2+, fe 3+). You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web so, [ar] can be written instead of 1s^2 2s^2 2p^6 3s^2 3p^6. Energy this problem has been solved! The charge is equal to the number of electrons that must be gained to fill the s and p. Web find the atomic number of. Note that the total number of electrons in the neutral atom adds up to the atomic number, so 2+2+6+2+6 = 18, which is the atomic number of ar. The charge is equal to the number of electrons that must be gained to fill the s and p. Energy this problem has been solved! Web what is the electron configuration of:. Web draw the electron configuration for a neutral atom of iron. Web most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill its outer s and p orbitals, thereby reaching the electron configuration of the next noble gas. Web electron configurations describe where electrons are located around the nucleus of an atom. Web therefore the. Web iron ion (fe 2+, fe 3+) electron configuration. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Web most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill its outer s and p orbitals, thereby reaching the. Otherwise, write the order of the energy levels with electron configuration chart: Web find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Web what is the electron configuration of: Web therefore the iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Therefore, the valence electrons of iron are eight. Both of the configurations have the correct numbers of electrons in each orbital, it is just a matter of how the electronic configuration notation. Web most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill its outer s and p orbitals, thereby reaching the electron configuration of the next noble gas. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web draw the electron configuration for a neutral atom of iron. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Web electron configurations describe where electrons are located around the nucleus of an atom. Thus, it is simple to determine the charge on such a negative ion: Web what is the electron configuration of: Web so, [ar] can be written instead of 1s^2 2s^2 2p^6 3s^2 3p^6. Web therefore the iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Energy this problem has been solved! Web iron ion (fe 2+, fe 3+) electron configuration. 1 s 2 2 s 2 2 p 3. Otherwise, write the order of the energy levels with electron configuration chart: Web for example, [ne] represents the 1s 2 2s 2 2p 6 electron configuration of neon (z = 10), so the electron configuration of sodium, with z = 11, which is 1s 2 2s 2 2p 6 3s 1, is written as [ne]3s 1:
How to Find the Valence Electrons for Iron (Fe)?

Draw the electron configuration for a neutral atom of iron. Quizlet
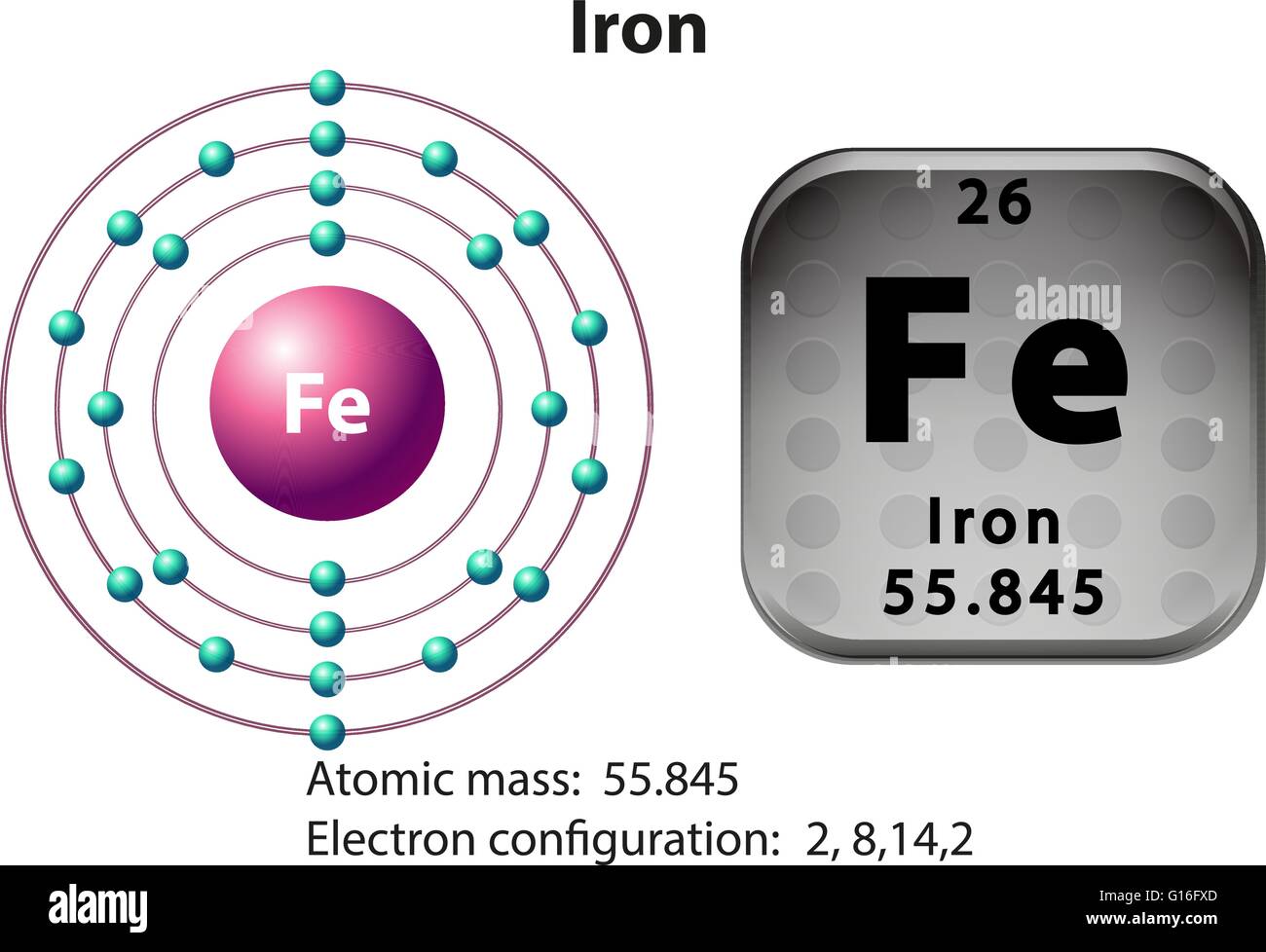
Symbol and electron diagram for Iron illustration Stock Vector Image

Write the Complete Electron Configuration for the Iron Atom
:max_bytes(150000):strip_icc()/Iron-58b602243df78cdcd83d3d5a.jpg)
Atom Diagrams Electron Configurations of the Elements
Solved Draw the electron configuration for a neutral atom of

Iron electronic configuration How to Write Iron electronic

Iron Protons Neutrons Electrons Electron Configuration

【5 Steps】Electron Configuration of Iron(Fe) Electron configuration

Electronic Configuration How To Write Electron ConfigurationChemistry
Note That When Writing The Electron Configuration For An Atom Like Fe, The 3D Is Usually Written Before The 4S.
The Ground State Electron Configuration Of Iron Is 1S 2 2S 2 2P 6 3S 2 3P 6 3D 6 4S 2.
Web Find The Atomic Number Of Nitrogen (7) And Use This Electron Configuration Calculator To Get A Complete Electron Configuration.
Note That The Total Number Of Electrons In The Neutral Atom Adds Up To The Atomic Number, So 2+2+6+2+6 = 18, Which Is The Atomic Number Of Ar.
Related Post: