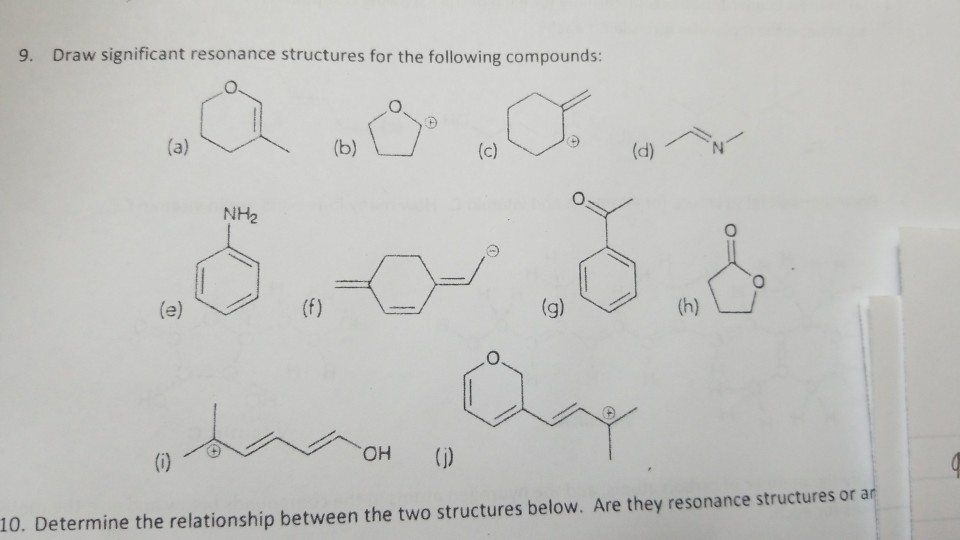
Draw Significant Resonance Structures For The Following Compound
Draw Significant Resonance Structures For The Following Compound - First, add curved arrow (s) to show the resonance using the following pattern: And so, this is called, pushing electrons, so we're moving electrons around, and it's extremely important to feel comfortable with moving electrons around, and being able to follow them. Practice problem 02.41 draw significant resonance structures for the following compound: A lone pair (on the second structure given. Draw significant resonance structures for the following compound. Use the concept of resonance to explain structural features of molecules and ions. (d) c6h5cho (e) (f) solution. View the full answer step 2. Modify the second structure given to draw the new resonance structure. O step 1 your answer is incorrect first, add curved arrow (s) to show the resonance using the following patterns a pi bond between two atoms of differing electronegativity, modify the second structure given to draw the new resonance structure. This means most atoms have a full octet. Draw all significant resonance structures for the following compound: Practice problem 02.52e draw all significant resonance structures for the following compound: Web chemistry questions and answers. Resonance is a mental exercise and method within the valence bond theory of bonding that describes the delocalization of electrons within molecules. The resonance structure that carries a. By following step 6 in the lewis structure drawing procedure, the double bond can be built between the central c and any of the terminal o’s to generate three structures, and they all look “the. Include one pins and charges in your structure. Web draw the resonance structures of molecules or ions that exhibit. Draw all significant resonance structures for the following compound: The resonance structure that carries a. Web draw all significant resonance structures for the following compound: (a) the structure of c6h5oh is: First, add curved arrow (s) to show the resonance using the following pattern: O step 1 your answer is incorrect first, add curved arrow (s) to show the resonance using the following patterns a pi bond between two atoms of differing electronegativity, modify the second structure given to draw the new resonance structure. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. Web as we’ve seen in previous. (d) c6h5cho (e) (f) solution. Which of this is/are most significant resonance structures? Draw all significant resonance structures for the following compound: How stable are the positive charges? First, add curved arrow (s) to show the resonance using the following pattern: When one lewis structure is not enough. In resonance structures, the electrons are able to move to help stabilize the molecule. Draw significant resonance structures for the following compound. Web so, the fact that we can draw an extra resonance structure, means that the anion has been stabilized. Modify the second structure given to draw the new resonance structure. Web as we’ve seen in previous posts, four key factors that determine the importance of resonance structures in organic chemistry are: To estimate average bond orders and average bond lengths. Web resonance structures are used when a single lewis structure cannot fully describe the bonding; Determine the relative stability of resonance structures using a set of rules. How stable are. Draw significant resonance structures for the following compound: Determine the relative stability of resonance structures using a set of rules. Draw all significant resonance structures for the following compound: When one lewis structure is not enough. Include one pins and charges in your structure. Modify the second structure given to draw the new resonance structure. O step 1 your answer is incorrect first, add curved arrow (s) to show the resonance using the following patterns a pi bond between two atoms of differing electronegativity, modify the second structure given to draw the new resonance structure. Draw all significant resonance structures for the following compound:. Resonance structures show the delocalization of electrons in a structure. Practice problem 02.52e draw all significant resonance structures for the following compound: A lone pair next to a pi bond. Resonance is a mental exercise and method within the valence bond theory of bonding that describes the delocalization of electrons within molecules. Use the concept of resonance to explain structural. Figure 1.3a versions of the carbonate anion lewis structure. To estimate average bond orders and average bond lengths. Draw all possible resonance structures for the following free radical: Web first, add curved arrow(s) to show the resonance using the following pattern: A lone pair next to a pi bond. When one lewis structure is not enough. O step 1 your answer is incorrect first, add curved arrow (s) to show the resonance using the following patterns a pi bond between two atoms of differing electronegativity, modify the second structure given to draw the new resonance structure. Consider ozone (o 3) solution. A lone pair next to a pi bond. View the full answer step 2. Draw all significant resonance structures for the following compound:step 1x your answer is incorrect.first, add curved arrow (s) to show the resonance using the following pattern: Draw significant resonance structures for the following compound. In resonance structures, the electrons are able to move to help stabilize the molecule. First, add curved arrow (s) to show the resonance using the following pattern: To understand the concept of resonance. We need the structure or the name of the compound to draw its resonance structures.[Solved] 5. Draw all significant resonance structures of

Solved Draw significant resonance structures for the
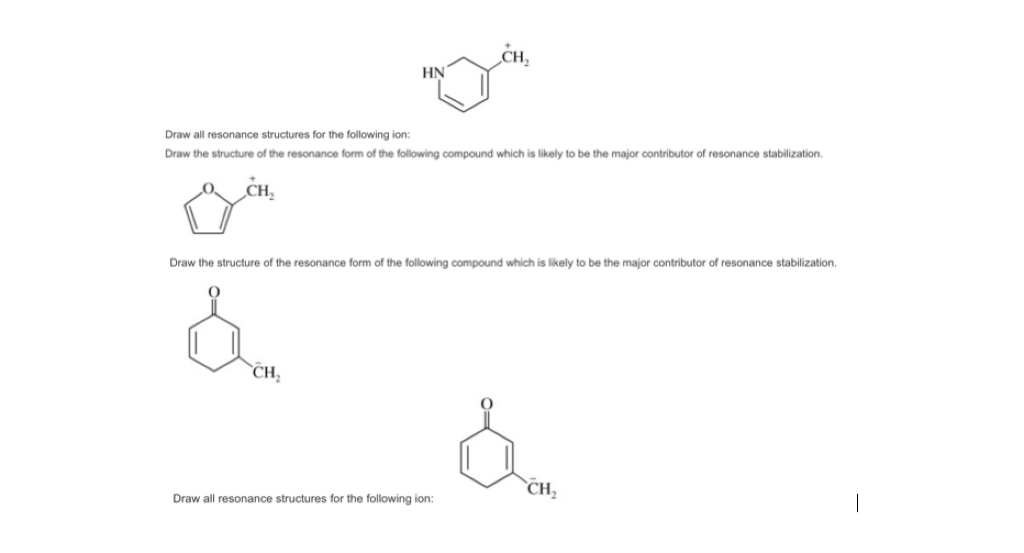
Draw Significant Resonance Structures for the Following Compound
Solved Draw significant resonance structures for the
[Solved] Draw significant resonance structures for the fo
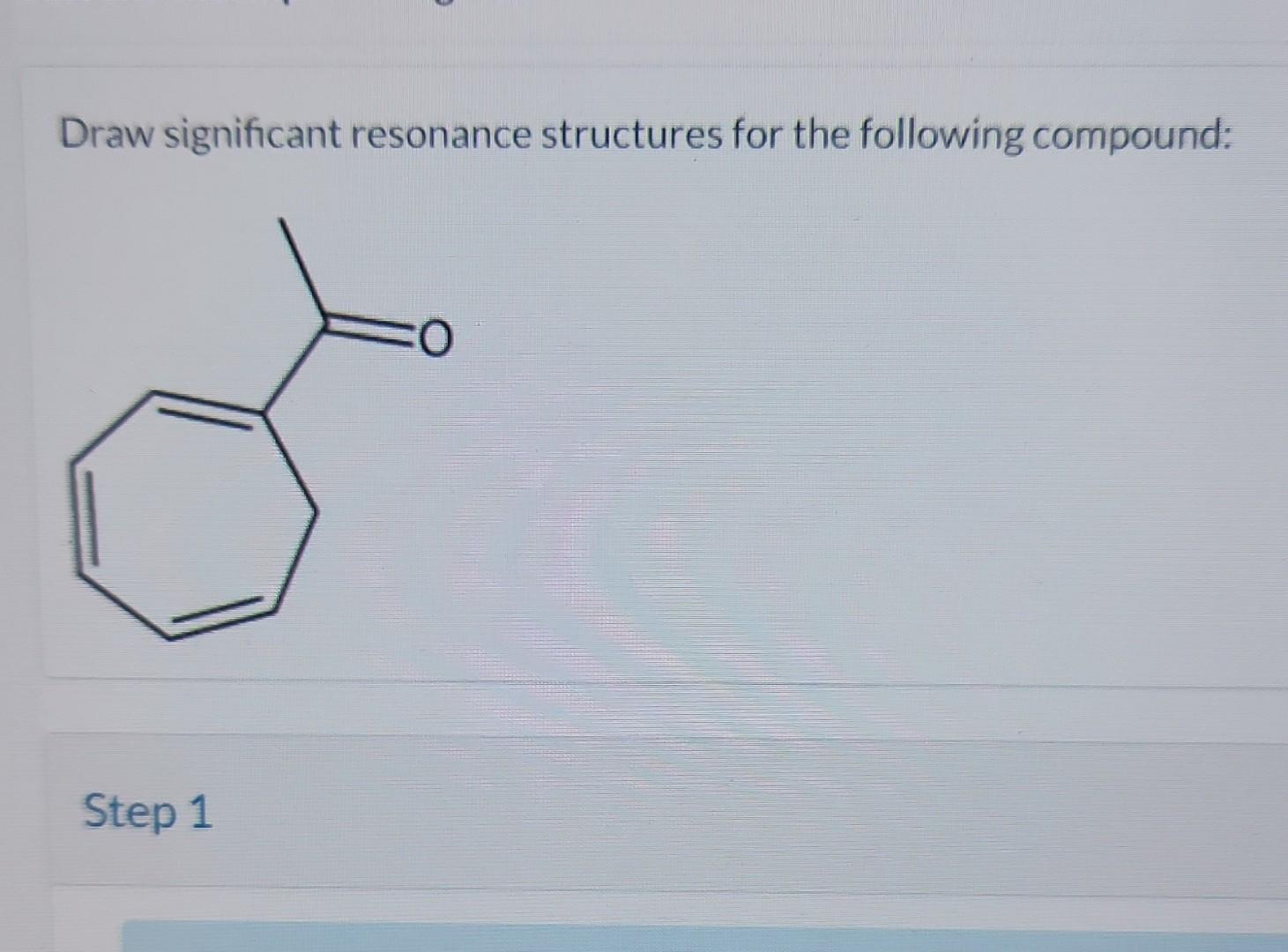
Solved Draw significant resonance structures for the
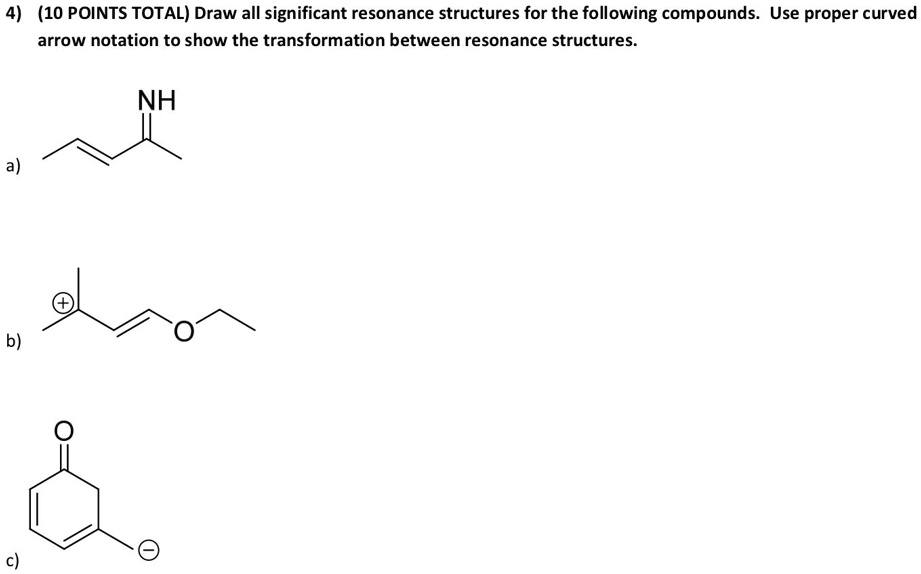
SOLVED4) (10 POINTS TOTAL) Draw all significant resonance structures
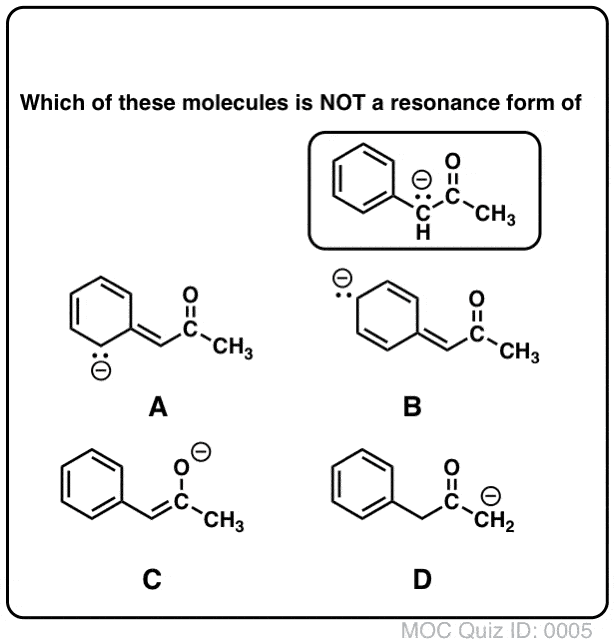
Draw Significant Resonance Structures for the Following Compound
draw significant resonance structures for the following compound
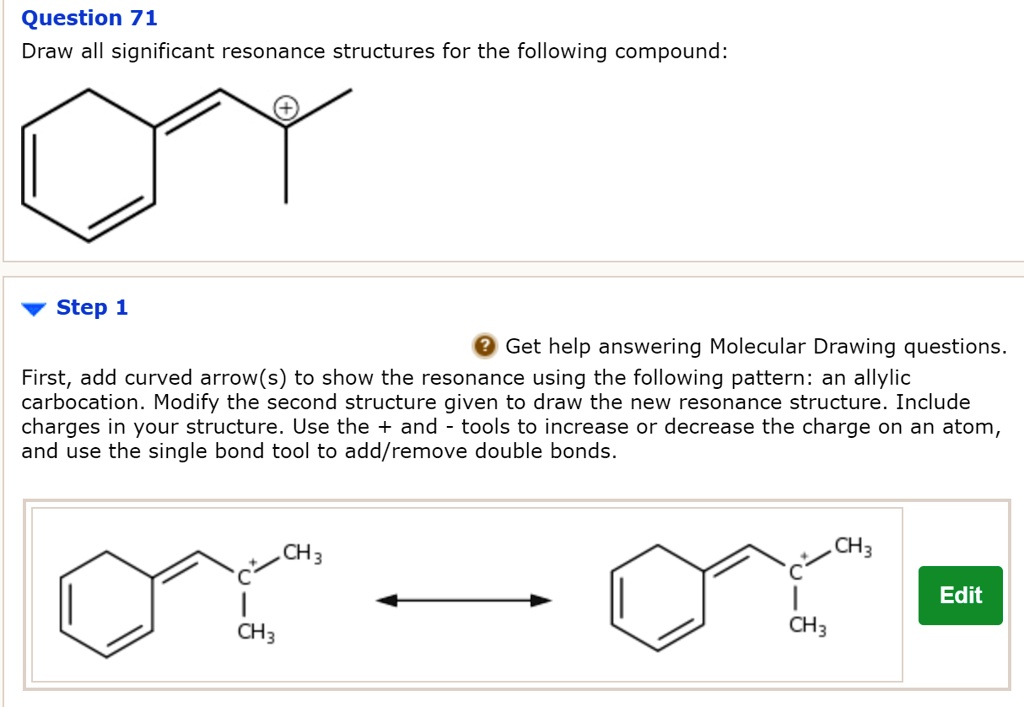
SOLVED Question 71 Draw all significant resonance structures for the
In The Example Below Structure A Has A Carbon Atom With A Positive Charge And Therefore An Incomplete Octet.
Determine The Relative Stability Of Resonance Structures Using A Set Of Rules.
Web Chemistry Questions And Answers.
Step 1 X Your Answer Is Incorrect.
Related Post:
![[Solved] 5. Draw all significant resonance structures of](https://media.cheggcdn.com/media/fcd/fcd9f0bb-7eb4-49f6-8362-879d3af3cd08/phpB9LMLQ)
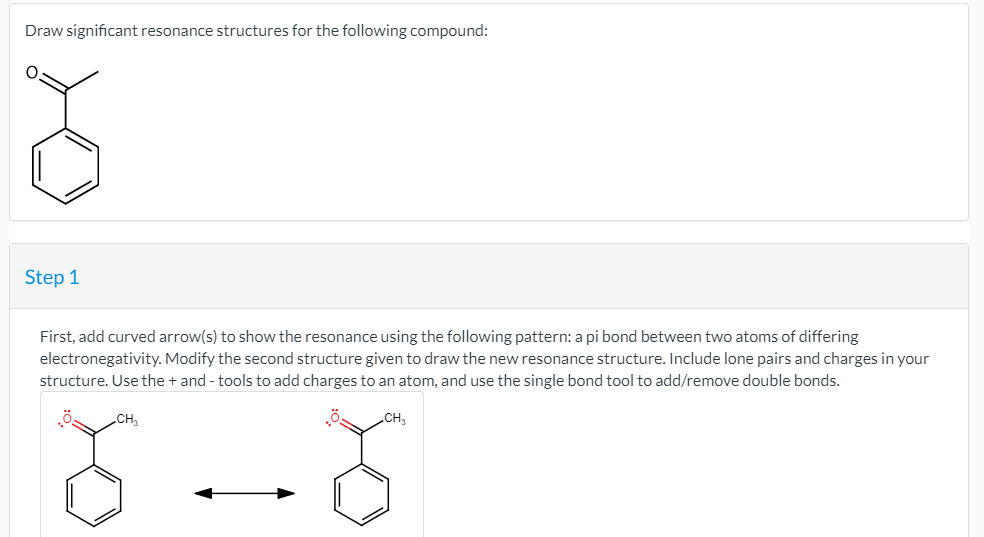
![[Solved] Draw significant resonance structures for the fo](https://media.cheggcdn.com/media/32e/32e698e1-d946-4ea6-aa6b-e1088a09bf45/phpDyLWm7)