Draw And Label Water Molecule
Draw And Label Water Molecule - Draw dotted lines showing where hydrogen bonding occurs. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. 84k views 11 years ago. Web because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: It is a simple molecule, consisting of just one oxygen atom and two hydrogen atoms. The negative charge of the electron is balanced by the positive charge of one proton in the hydrogen nucleus. Web a molecule of water is composed of two atoms of hydrogen and one atom of oxygen. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. This diagram is based on calculations and comparison to experiments. The diagram below depicts a collection of water molecules. The plus end of one—a hydrogen atom—associates with the minus end of another—an oxygen atom. Surface tension, heat of vaporization, and vapor pressure. Click the card to flip 👆. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Your first diagram should be an electron distribution model (showing the atomic nuclei and the. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Web #tutorials #chemistry #lessonsin this video, we will draw dot and cross diagram of water molecule. Your first diagram should be an electron distribution model (showing the atomic nuclei and the. Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. There are two lone pairs of electrons on. Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Hydrogen bonding of water molecules. 84k views 11 years ago. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). Web #tutorials #chemistry #lessonsin this video, we will draw dot and cross diagram of water molecule. Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Web strong bond within one water molecule when electrons. Web complete the labels showing the locations of the hydrogen atoms, the oxygen atom, and the regions of positive and negative charge. Remember that a hydrogen atom has one proton and one electron. It is a simple molecule, consisting of just one oxygen atom and two hydrogen atoms. Web identify three special properties of water that make it unusual for. Click the card to flip 👆. Web the water molecule, visualized three different ways: The second should be a. The atomic structure of water. Web water is classified as a polar molecule because of its polar covalent bonds and its bent shape 2, 3. There are two lone pairs of electrons on. From there, we will determine the structural formula of wa. Web molecular structure of water: This diagram is based on calculations and comparison to experiments. Click the card to flip 👆. Web strong bond within one water molecule when electrons are unequally shared. Students will be able to explain, on the molecular level, what makes water a polar molecule. Web draw a water molecule with charges. Boiling point and freezing point. Web draw and label a ball and stick presentation of a water molecule. Web the water molecule, visualized three different ways: Water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond. Web draw a water molecule with charges. The one and only electron ring around the nucleus of each hydrogen atom has only one electron. The second should be a. It is one of the easiest atoms to build a model of, and is therefore an excellent starting point for students learning to build molecular models. There are two lone pairs of electrons on. The diagram below depicts a collection of water molecules. This problem has been solved! Identify three special properties of water that make it unusual for a. Web example 1 1: Students will be able to explain, on the molecular level, what makes water a polar molecule. The diagram below depicts a collection of water molecules. The one and only electron ring around the nucleus of each hydrogen atom has only one electron. Web chemistry of life > structure of water and hydrogen bonding. The plus end of one—a hydrogen atom—associates with the minus end of another—an oxygen atom. Water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond. The atomic structure of water. Draw dotted lines showing where hydrogen bonding occurs. Describe the structure, such as it is, of. This diagram is based on calculations and comparison to experiments. It is a simple molecule, consisting of just one oxygen atom and two hydrogen atoms. Web molecular structure of water: Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. This problem has been solved! Web simon fraser university.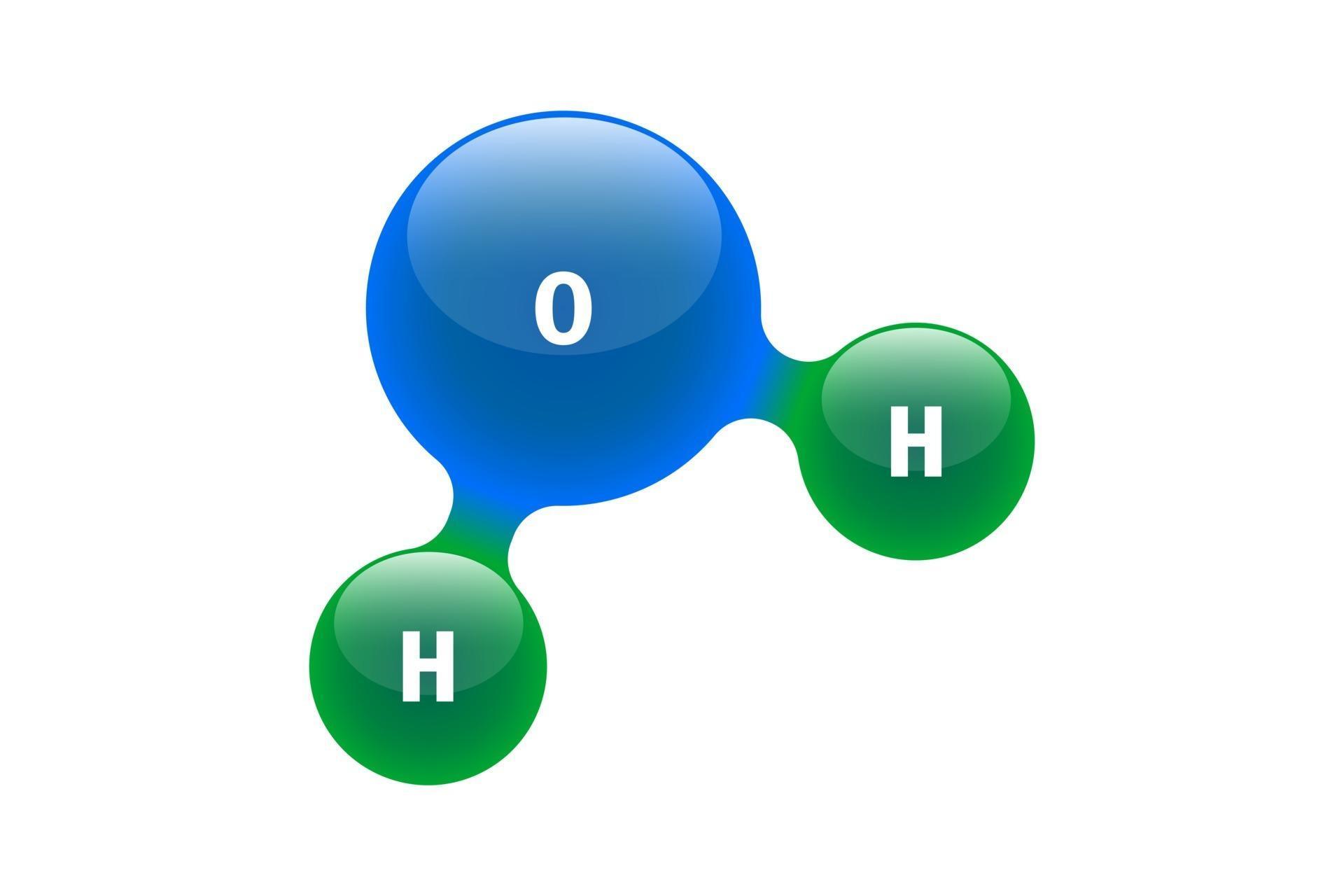
Chemistry model of molecule water H2O scientific elements. Integrated
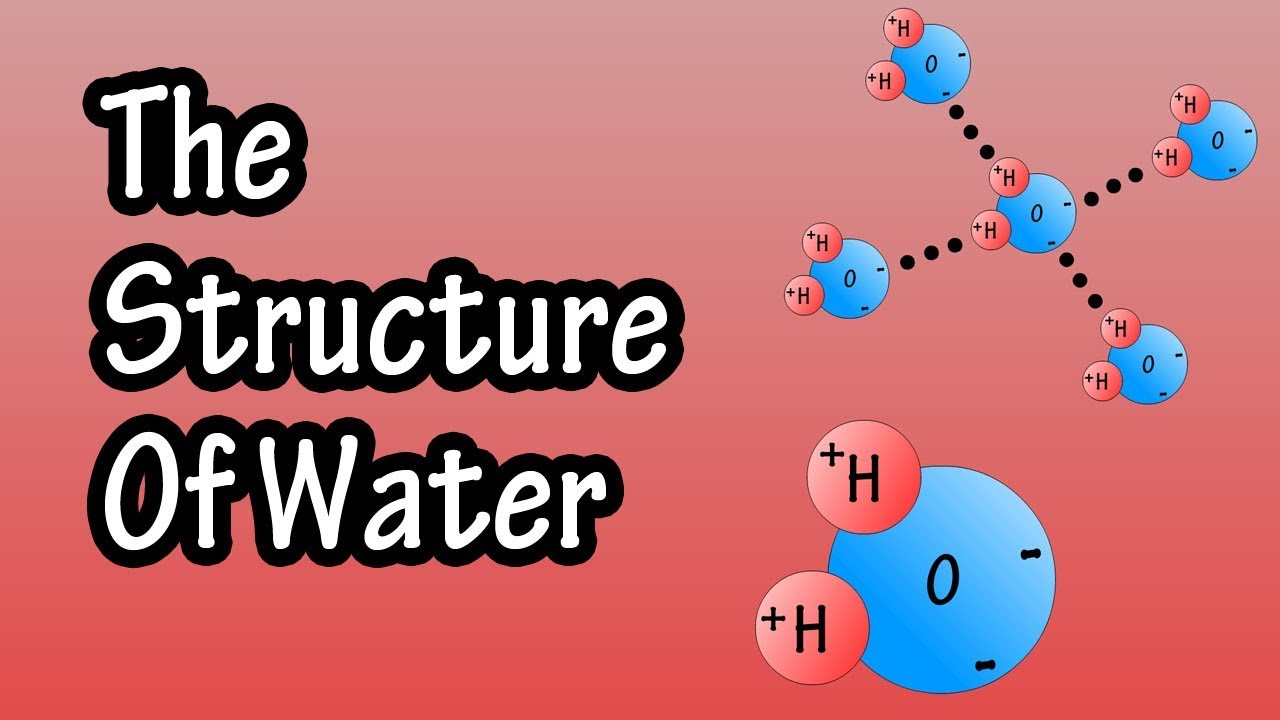
Structure Of Water Molecule Chemistry Of Water Properties Of Water
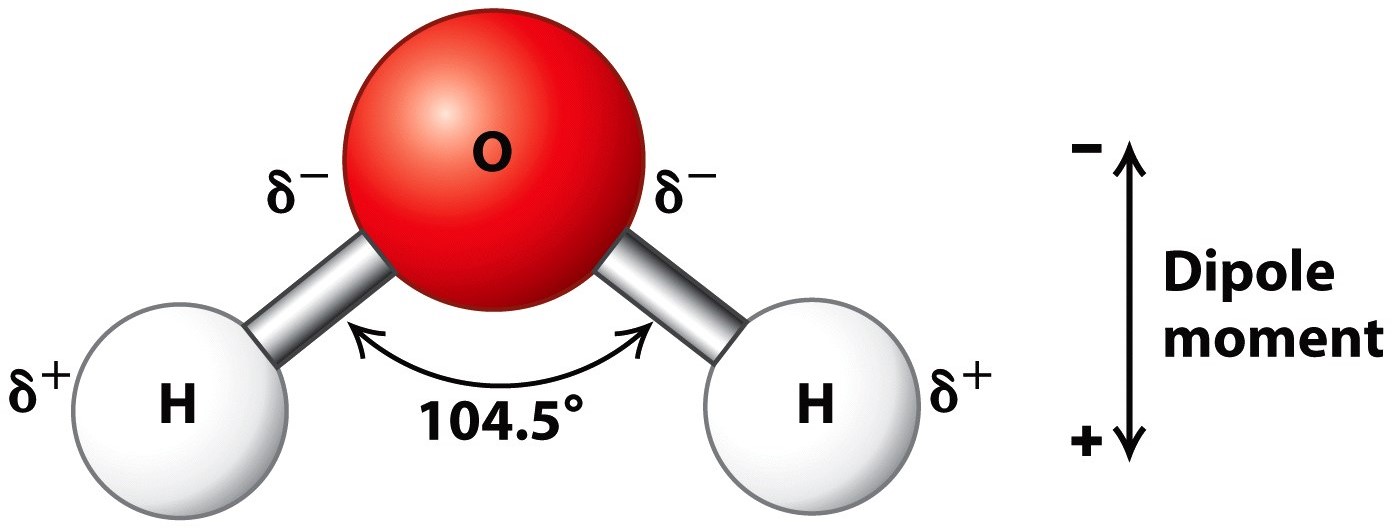
Describe the Structure of a Water Molecule
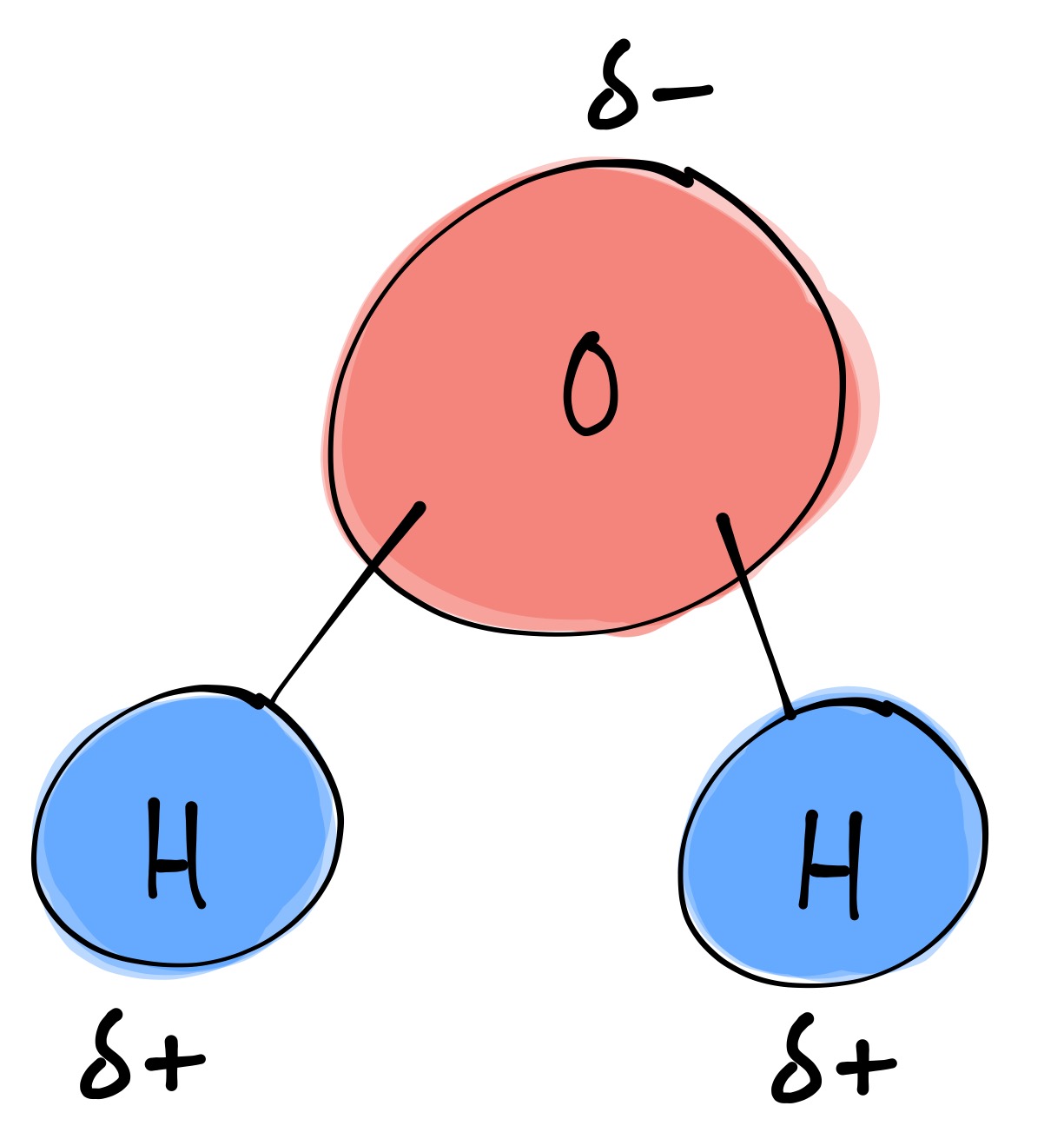
bi·ol·o·gy (bīˈäləjē) Structure of a Water Molecule

Diagram Of Water Molecule
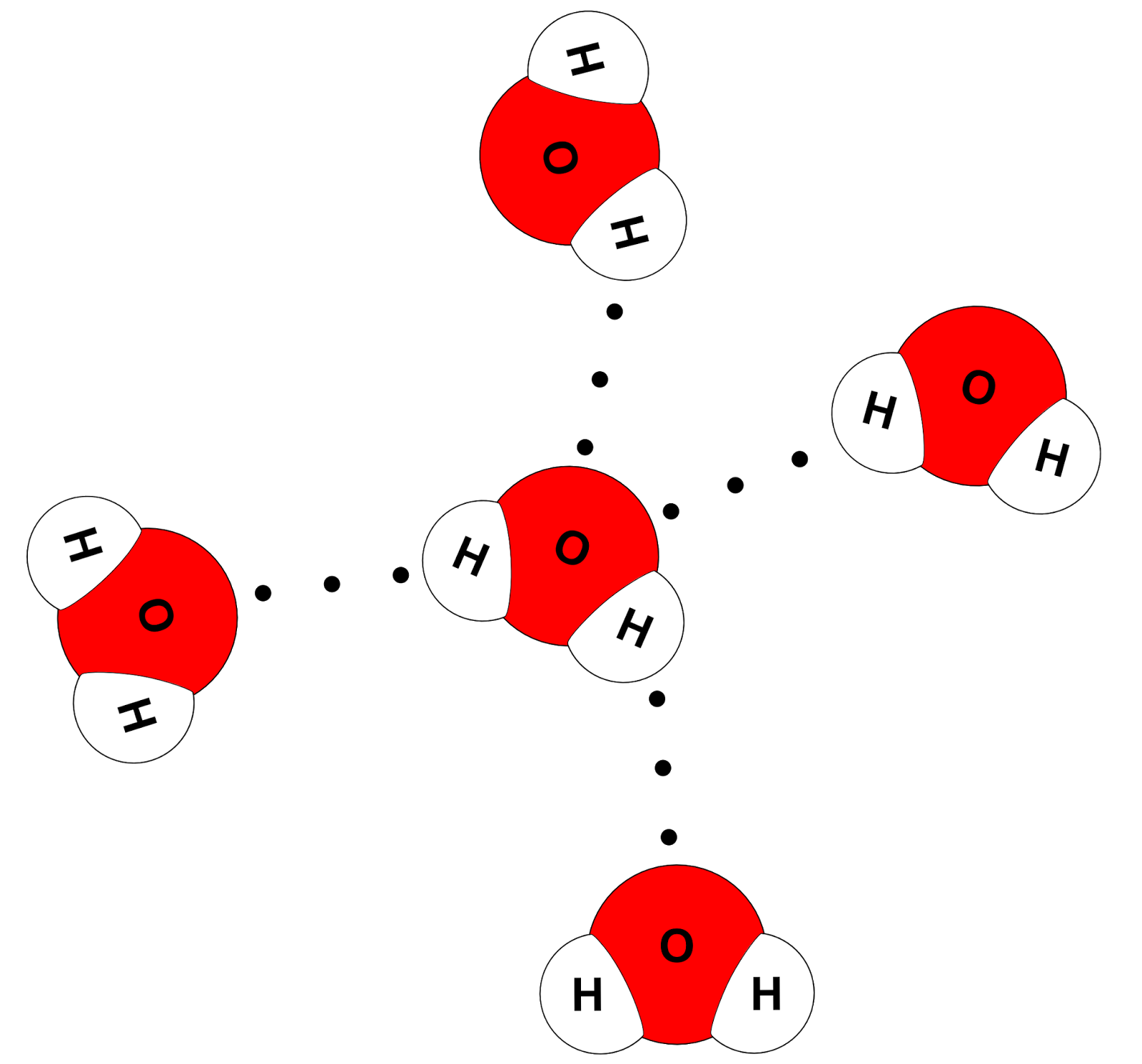
Science online The importance of the water and its structure

Draw a neat well labelled diagram of information of water molecule
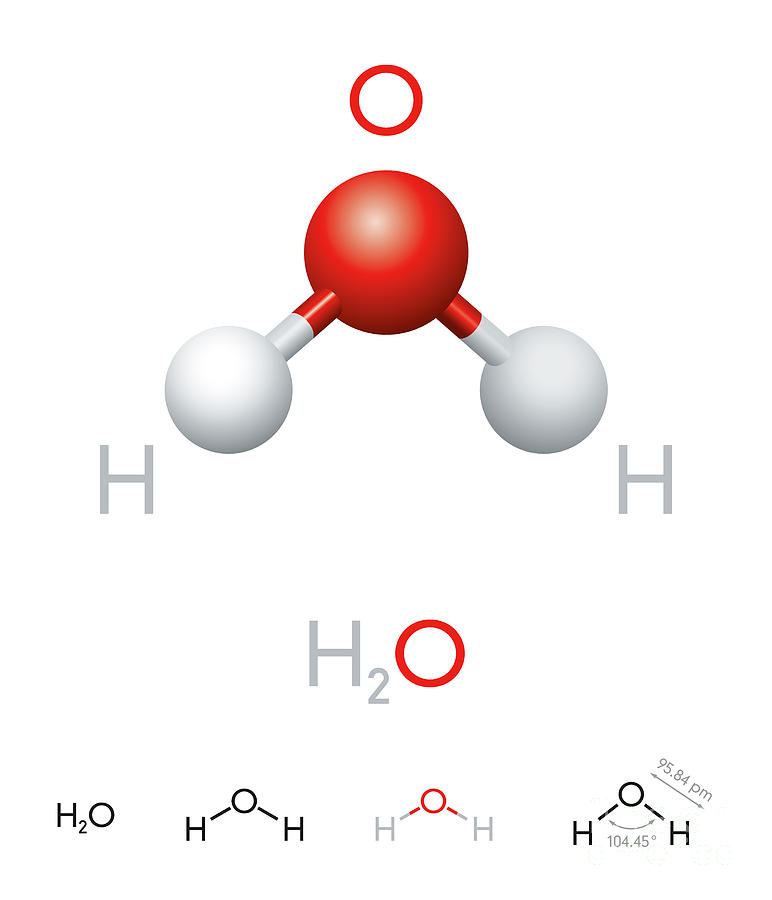
H2O Water molecule model and chemical formula Digital Art by Peter
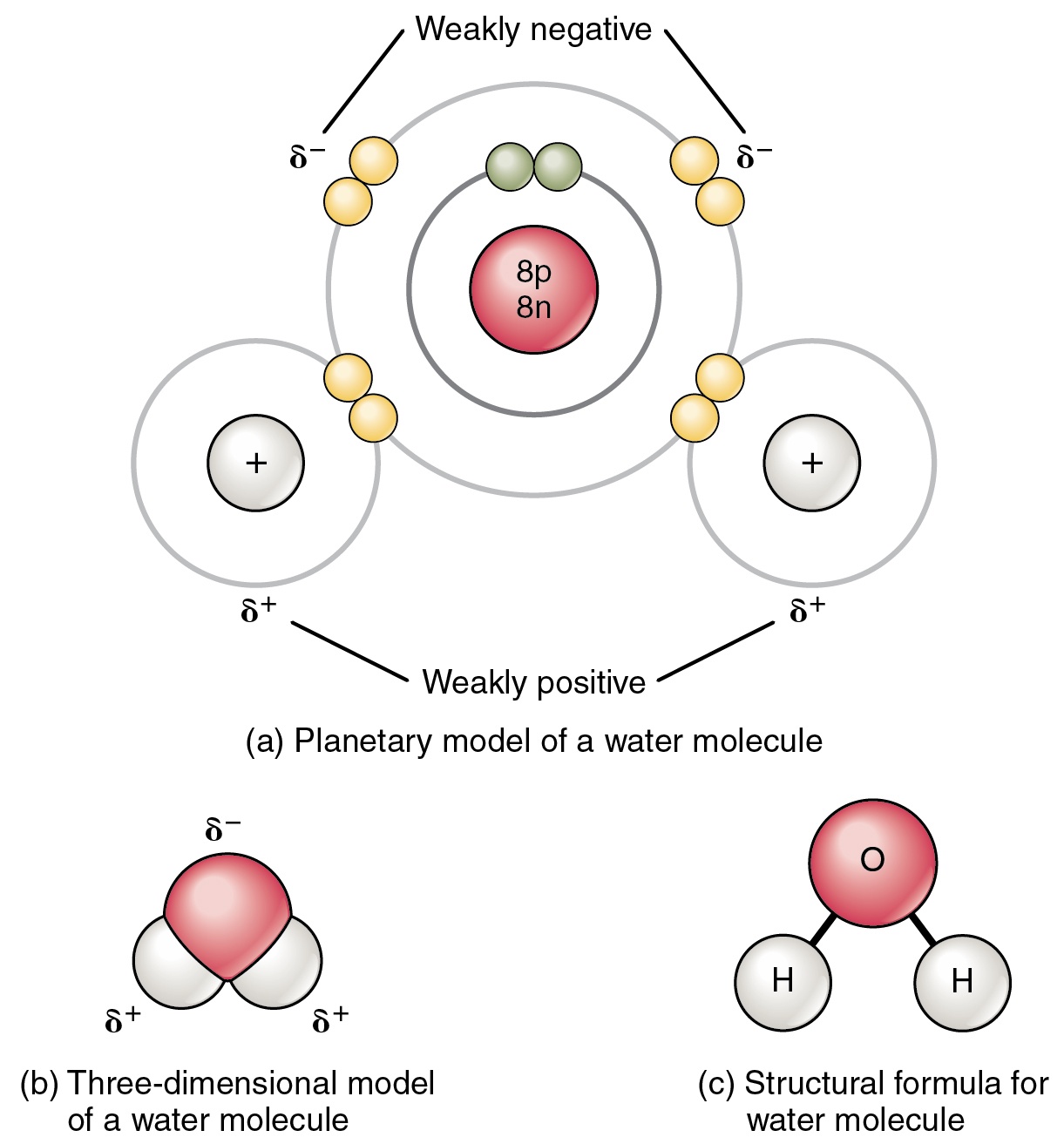
Water — Molecular Structure & Bonding Expii

Water, Water Everywhere… Science, POLITICS, & Religion
Explain What Is Meant By Hydrogen Bonding And The Molecular Structural Features That Bring It About.
Because Of The Higher Electronegativity Of The Oxygen Atom, The Bonds Are Polar Covalent ( Polar Bonds ).
84K Views 11 Years Ago.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Related Post: