Capa Form Template
Capa Form Template - Web capa plans must be thorough and well documented. Web corrective and preventive action (capa) form template includes four main components of a capa form. Capa reports are typically initiated in response to customer complaints, internal audits, or other quality events. [irb] [name, title, and the site or institutional affiliation of the. A capa process is a systematic approach used by companies to address corrective and preventive actions (capa). What is corrective and preventive action? There are different templates you can use including product redesigns, training, and other improvements. Corrective and preventive actions (capa) inspectional objectives. Web get started with this customizable capa form template. Track progress, manage resources, and improve communication with cascade, the leading strategy execution platform. Web corrective action and preventive action (capa) plan template. Sop corrective and preventive action (capa) templates iso 13485 templates. If you’ve ever had a workplace incident, you should know how to create a capa plan. Web free lean six sigma templates. A capa report is documented evidence that a capa system has been successfully implemented. Corrective and preventive actions (capa) form a key approach in quality management systems. Download our free capa form! They provide a structured approach to managing and resolving issues as they arise. Download our accompanying white paper for more detail into each. Download corrective and preventative action plan form template_2019.11.13. When it’s time to address the real issue, whether it’s harassment, fraud, or breaking your company’s code of ethics, where do you begin? Web free lean six sigma templates. They provide a structured approach to managing and resolving issues as they arise. There are also different ways to implement corrective actions. This is a free template, provided by openregulatory. Web what is a capa process? They provide a structured approach to managing and resolving issues as they arise. Sop corrective and preventive action (capa) templates iso 13485 templates. Web staying on the journey to success: Investigator assigned and target due date; There are also different ways to implement corrective actions. Paperless solutions5 star ratedform search enginefast, easy & secure (capa) we’ve covered medical device capa examples,. Web there are two components to the corrective actions and preventive actions (capa) form. Track progress, manage resources, and improve communication with cascade, the leading strategy execution platform. By andy marker | january 30, 2018 (updated july 21, 2021) experience, training, and sound procedures in your facility may mean that problems rarely arise. [irb] [name, title, and the site or institutional affiliation of the. Investigator assigned and target due date; Download corrective and preventative action plan form template_2019.11.13. Download our free capa form! Web the capa (corrective and preventive action) form template by wpforms is essential for organizations to address and rectify issues systematically. Corrective and preventive actions (capa) form a key approach in quality management systems. How does the capta form template work? Download our accompanying white paper for more detail into each. In your plan, include information that is: Investigator assigned and target due date; Web there are two components to the corrective actions and preventive actions (capa) form. Download corrective and preventative action plan form template_2019.11.13. Click here to download 3 free templates for capa, nonconformances, and complaints. This capa form template provides fields for all of the key information you need to document as part of your. A capa report is documented evidence that a capa system has been successfully implemented. This can be used by compliance officers when formulating a corrective action to resolve the issue, and a preventive action to lower the risk of its recurrence. Web the capa (corrective and preventive action) form template by wpforms is essential for organizations to address and rectify. Web staying on the journey to success: There are different templates you can use including product redesigns, training, and other improvements. Web corrective and preventive action (capa) form template includes four main components of a capa form. Corrective and preventive actions (capa) inspectional objectives. By andy marker | january 30, 2018 (updated july 21, 2021) experience, training, and sound procedures. Web what is a capa report? They provide a structured approach to managing and resolving issues as they arise. Sop corrective and preventive action (capa) templates iso 13485 templates. Download our accompanying white paper for more detail into each. [date that the capa is written] to: How capa can sustain your organization. Web get started with this customizable capa form template. Download corrective and preventative action plan form template_2019.11.13. In your plan, include information that is: Click here to download 3 free templates for capa, nonconformances, and complaints. A capa process is a systematic approach used by companies to address corrective and preventive actions (capa). Root cause analysis and action plan; Capa reports are typically initiated in response to customer complaints, internal audits, or other quality events. A capa report is documented evidence that a capa system has been successfully implemented. Download our free capa form! There are different templates you can use including product redesigns, training, and other improvements.
Corrective and preventive action plan CAPA report form
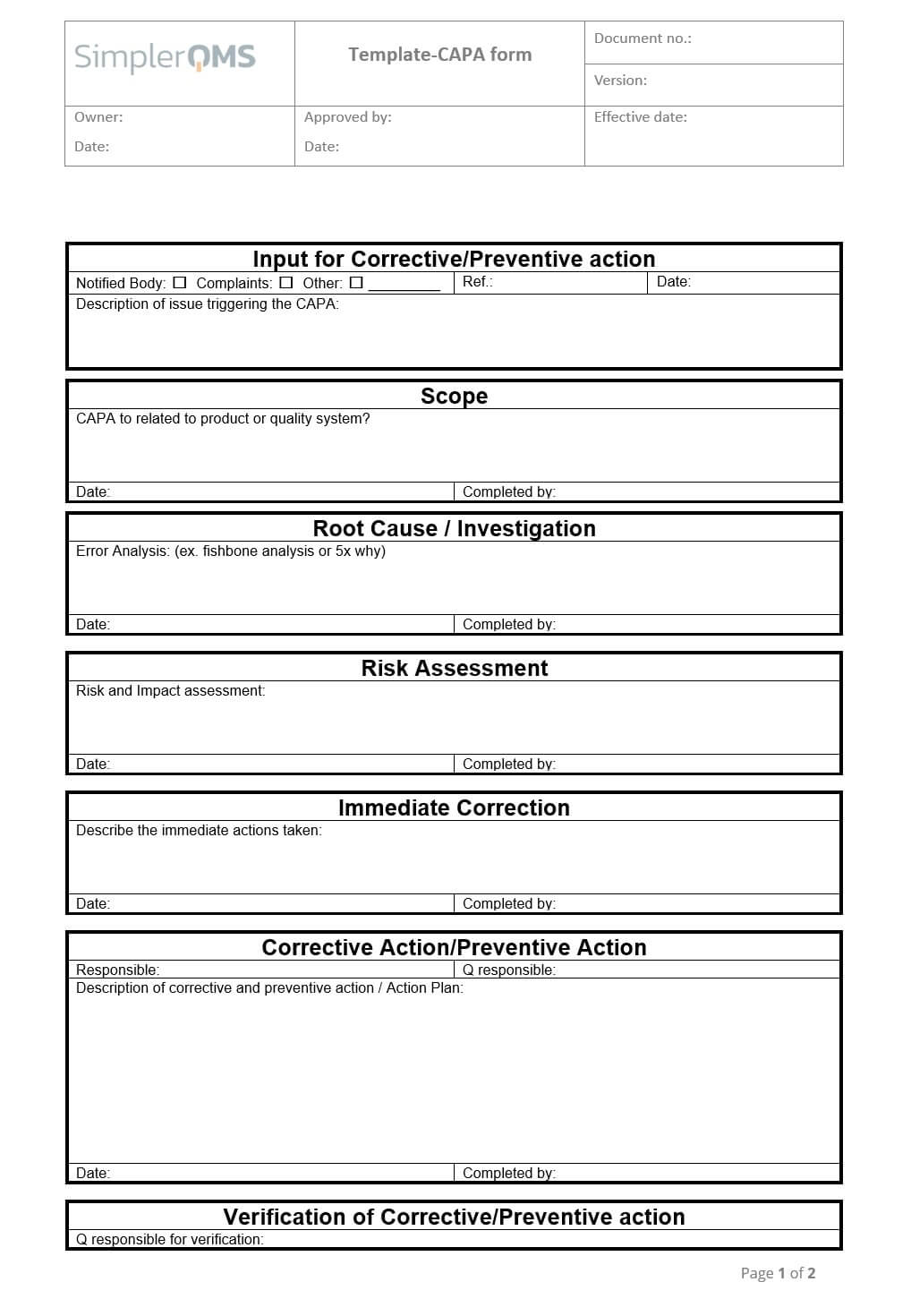
Corrective and Preventive Action (CAPA) Form Template SimplerQMS
CAPA Form Example

CAPA form Corrective action and preventive action
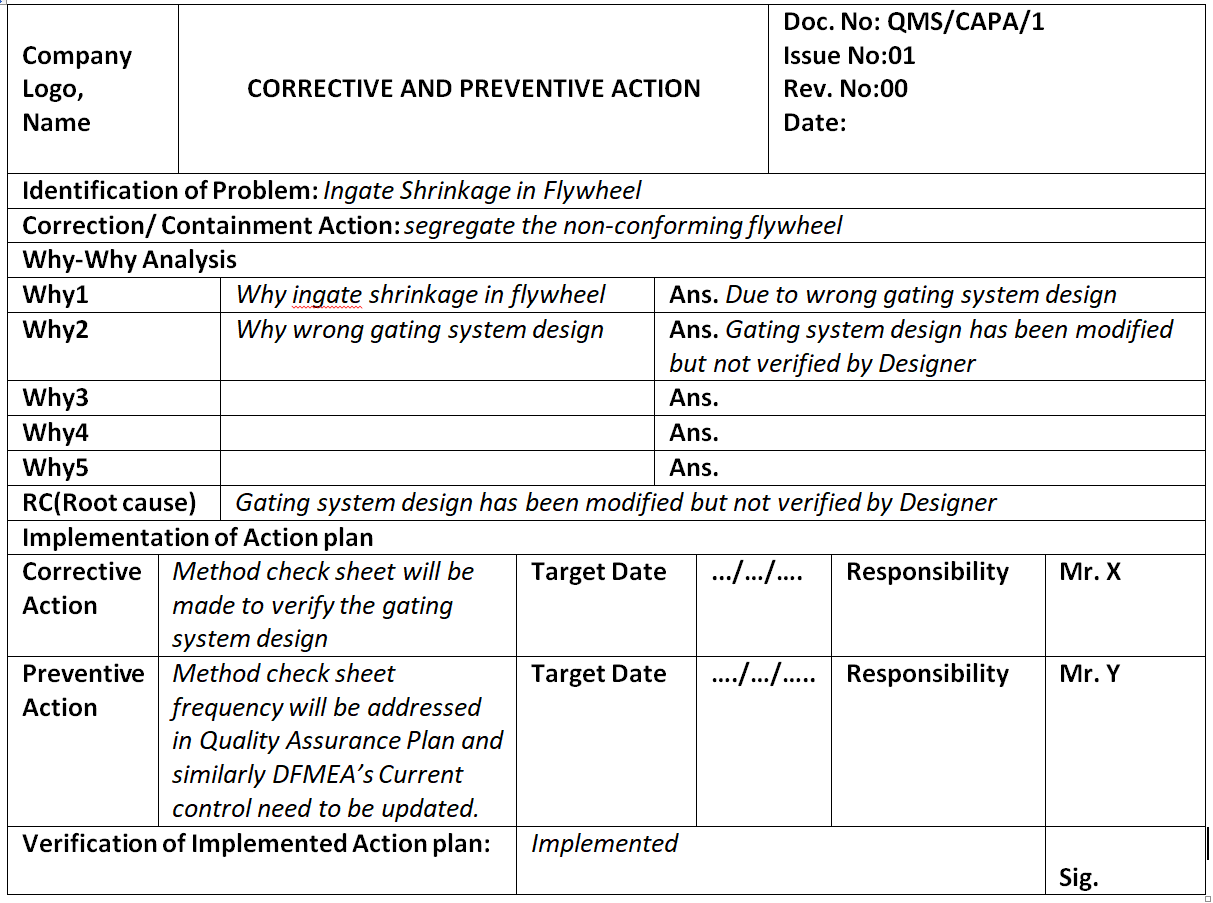
Corrective And Preventive Action Plan Template
Capa Form Template Free Printable Templates

Sample Capa form Beautiful Corrective Action Report Example Action
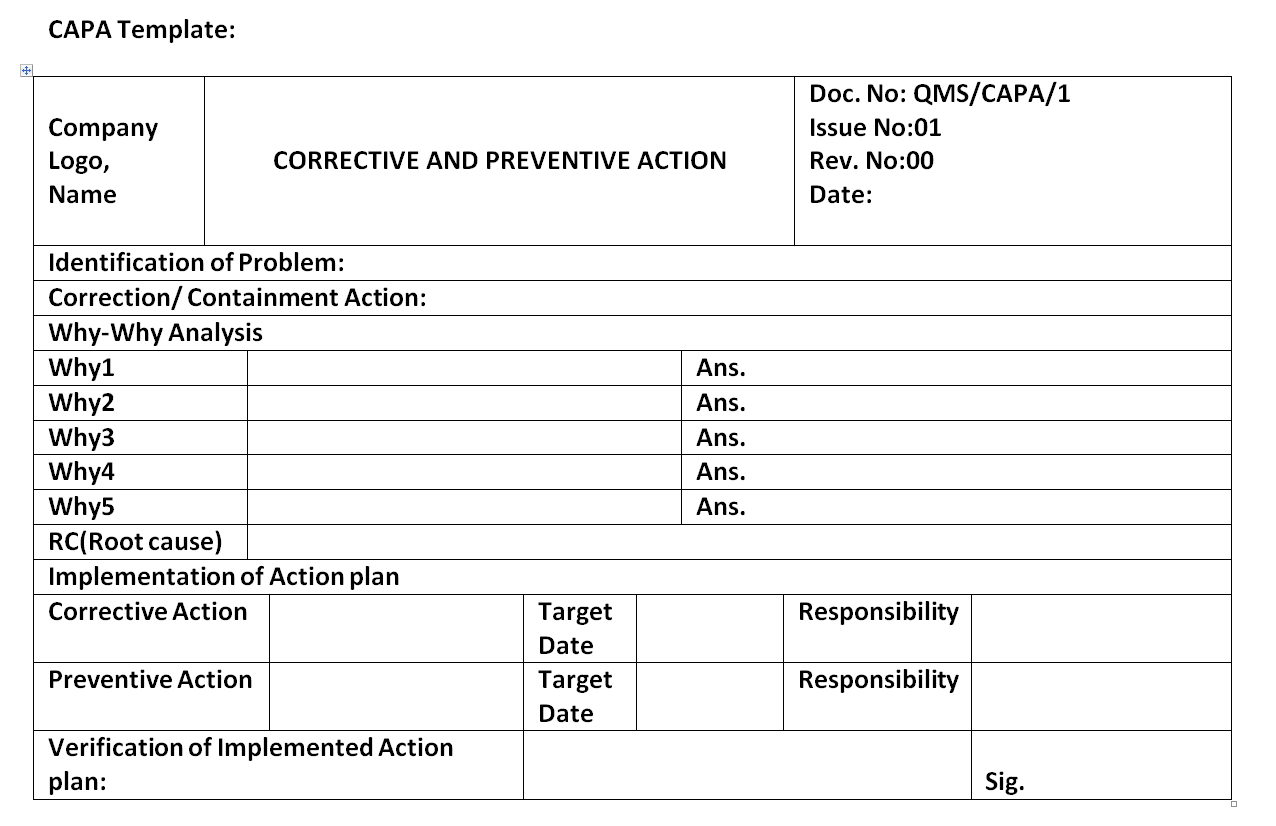
Corrective and Preventive Action Format CAPA with Example

Capa Form Template Free Free Sample, Example & Format Templates

How to implement Corrective action Preventive action?
This Can Be Used By Compliance Officers When Formulating A Corrective Action To Resolve The Issue, And A Preventive Action To Lower The Risk Of Its Recurrence.
Web There Are Two Components To The Corrective Actions And Preventive Actions (Capa) Form.
Guidance Information On Completing The Capa Form With A Sample Capa Can Be Found Below To Assist In Completion.
Both Corrective And Preventive Actions Are Often Used Within Other Methodologies, Such As 8D.
Related Post:

